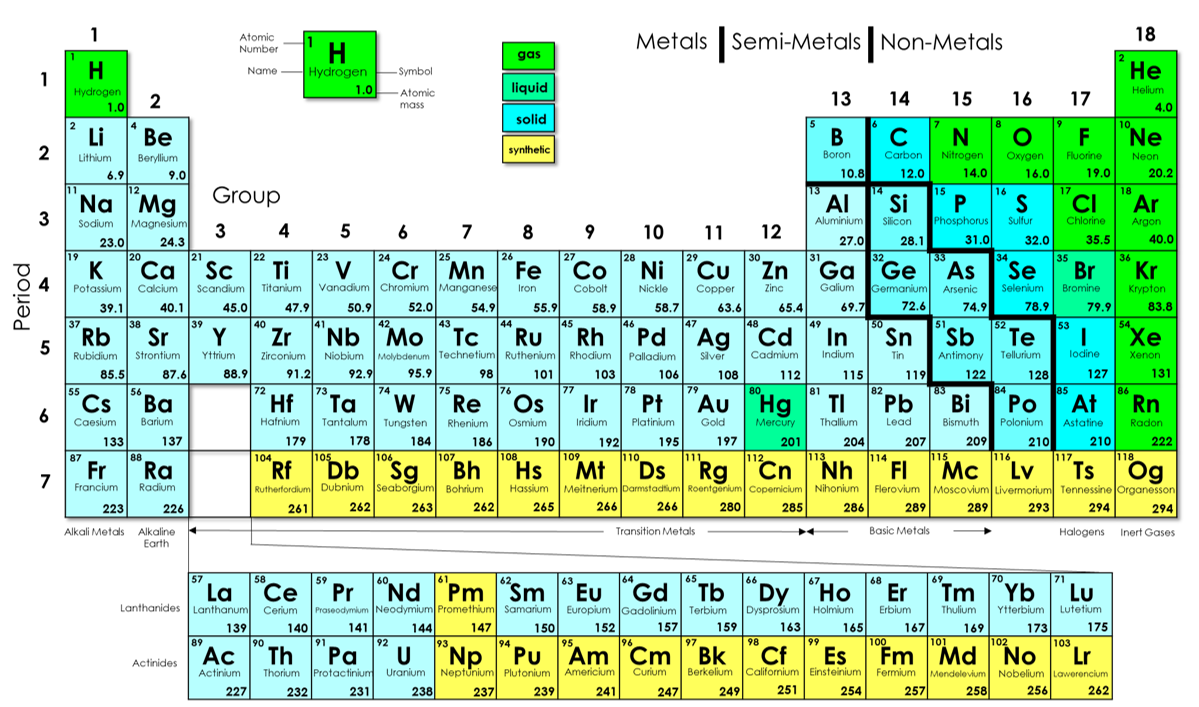
What are the horizontal lines on the periodic table called?
The horizontal rows are called period and the vertical columns are called groups. What element is soft and shiny? Group 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
What do the rows mean on the periodic table?
What do periodic table rows mean? Each row represents one period; the period number of an element indicates how many of its energy levels house electrons. Sodium, for instance, sits in the third period, which means a sodium atom typically has electrons in the first three energy levels.
How is the periodic table arranged horizontally?
- Definition: Each horizontal row of elements in the Periodic Table is known as a period.
- There are 7 horizontal rows of elements in the Periodic Table, known as Period 1, Period 2, until Period 7.
- Periods 1 to 3 are short periods while Periods 4 to 7 are long periods. ...
What are the names of the rows in the periodic table?
Key Takeaways: Parts of the Periodic Table The periodic table orders elements by increasing atomic number, which is the number of protons in the atom of an element. The rows of the periodic table are called periods. ... The columns of the periodic table are called groups. ... The three broad categories of elements are metals, nonmetals, and metalloids. ...
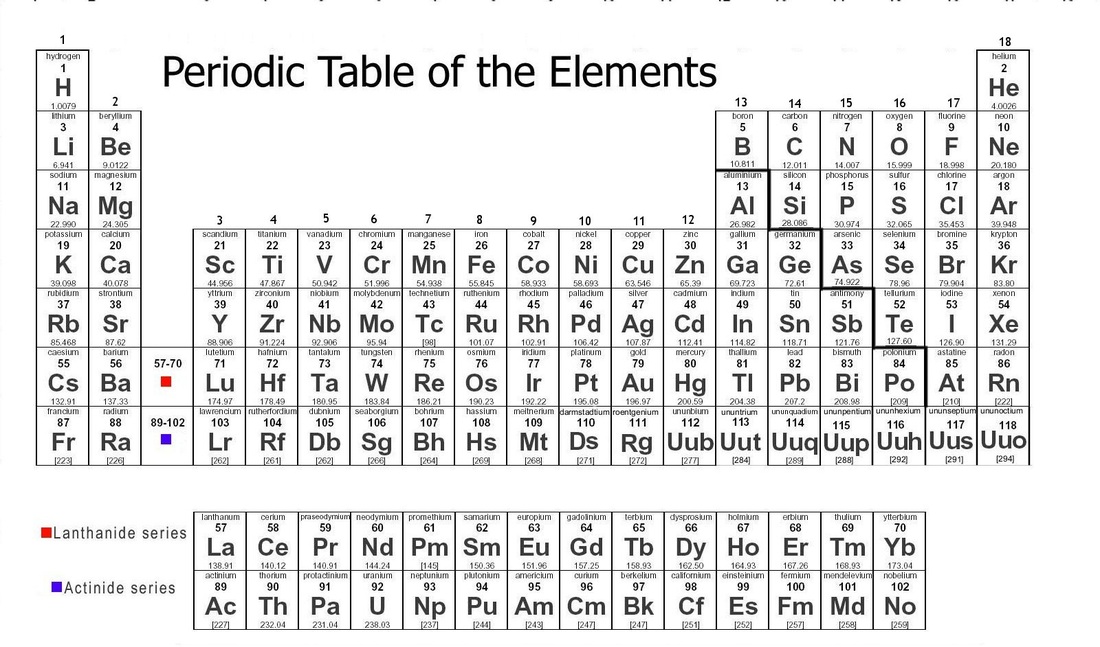
Why does the valance shell get closer to the nucleus?
The electrons are pull towards the nucleus and valance shell get closer to the nucleus. As a result of this greater nuclear attraction atomic radius decreases and ionization energy increases because it is very difficult to remove the electron from atom and more energy is required.The electron affinity also increases along period because of greater positive charge.
Why does the atomic size decrease in the same period of the periodic table?
The atomic size tend to decrease in same period of periodic table because the electrons are added with in the same shell. When the electron are added, at the same time protons are also added in the nucleus. The positive charge is going to increase and this charge is greater in effect than the charge of electrons. This effect lead to the greater nuclear attraction.