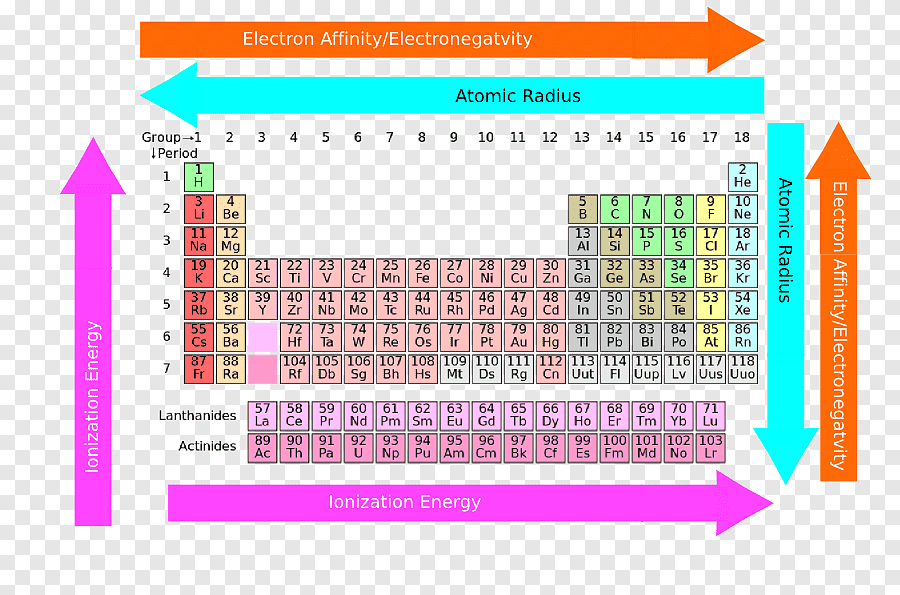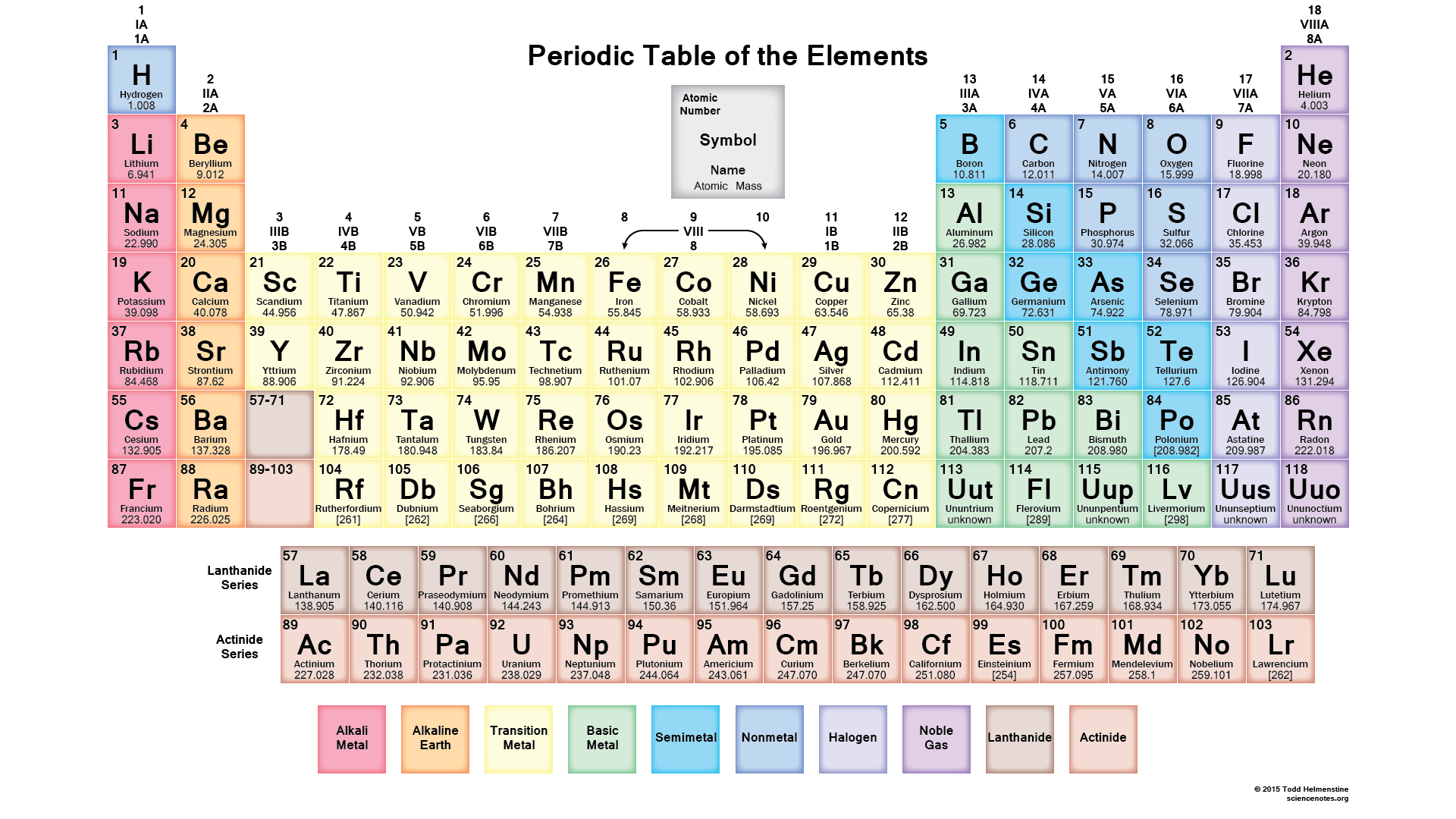
In the modern periodic table:
- The elements are arranged in order of increasing atomic number.
- The horizontal rows are called periods .
- The vertical columns are called groups .
- Elements in the same group are similar to each other.
How many elements can you find on a periodic table?
Unless you currently work in a field that involves science, the days of memorizing the elements of the period table are long behind you. There are currently 118 known elements on the periodic table, and we’ve put 20 of them below to test your smarts.
How do you find elements in the periodic table?
How do you find elements in the periodic table? To find the number of electrons an element has, locate it on the periodic table of elements, find the atomic number, and note the number of protons; because atoms are naturally electrically neutral, the protons and electrons are usually equal. Look at the oxidation number for further information.
How can elements be group in periodic table?
What are the three ways of grouping elements in the periodic table?
- Periodic Organization. In the periodic table, an element is defined by its vertical group and horizontal period.
- Scientific Rationale.
- Alkali and Alkaline Earth Metals.
- Transition Metals.
- Metalloids and Nonmetals.
- Noble Gases.
How did Mendeleev arrange elements in periodic table?
Mendeleev arranged the periodic table in order of increasing atomic weight of the elements. He determined that there was a pattern when he arranged these elements into horizontal rows. Mendeleev placed the elements into horizontal rows in his periodic table of elements.

What are the elements in the periodic table?
Quick look on Types of Elements on Periodic table 1 Metals#N#Alkali metals#N#Alkaline earth metals#N#Transition metals#N#Inner transition metals 2 Nonmetals 3 Metalloids 4 Families on Periodic table#N#Boron family#N#Carbon family#N#Nitrogen family#N#Chalcogens#N#Halogens#N#Noble gases
How many elements are in the modern periodic table?
In this way, all the 118 known elements are arranged in the Modern Periodic table based on their atomic number.
Why are there two rows at the bottom of the periodic table?
The two rows are placed at the bottom of the Periodic table in order not to distort the shape of Periodic table and also it makes the Periodic table fit perfectly on the A4 size paper.
What are the rows of a periodic table called?
The rows of the Periodic table are known as Periods.
What is the period table?
And it was named as Modern Periodic table. This Periodic table consists of vertical columns called groups and horizontal rows called periods. There are various types of elements on the Periodic table like metals, nonmetals, metalloids, etc. So this is how the Periodic table is arranged.
Where is the atomic number 57?
The element with atomic number 57 is placed in the main part of the Periodic table as shown in below image. Further the elements having atomic number 58, 59, 60, etc are placed in separate rows at the bottom of the Periodic table. This repeats upto element with atomic number 71.
How many groups are there in the periodic table?
There are total 18 groups in modern Periodic table.
How are the elements in the periodic table arranged?
The Periodic table elements are arranged in the increasing order of their atomic number. The arrangement of elements in the Periodic table starts from the very first top left corner. The first element with atomic number 1 (i.e hydrogen) is placed in the first cell, then gradually the elements with atomic number 2, 3, 4 upto 118, ...
Why is the periodic table arranged by atomic number?
Periodic table is arranged by Atomic number because of electrons present in the outermost orbit (which are responsible for the chemical properties of the elements.)
What is the relationship between the number of protons and the number of electrons?
What is a relationship between number of protons and number of electrons? Simple answer: In a neutral atom, the number of protons and number of electrons are equal. For example, this is a neutral helium atom. Here you can see that the helium atom has 2 protons and the number of electrons are also 2.
What does atomic number mean?
Atomic number = Number of electrons. The electrons present in the outermost orbit represent the chemical properties of the elements. Hence to classify the elements on the basis of similarities in their chemical properties, they are arranged in the Periodic table on the basis of atomic number. In other words, Atomic number indicates the number ...
What are electrons responsible for?
These electrons are responsible for the chemical properties of an element. Hence for grouping the elements according to the similar chemical properties, the elements of the Periodic table are arranged in the increasing order of their atomic number. Later on, we saw that there are groups as well as periods on the Periodic table.
Why are elements 58 to 71 and 90 to 103 in separate rows?
These elements are placed in separate rows at the bottom of the Periodic table because they differ in the chemical properties, plus by placing them at the bottom, ...
How many elements were known in 1869?
During the year 1869, only 63 elements were known. Mendeleev arranged these known elements in the increasing order of their atomic masses. And the Mendeleev’s Periodic table was something like this (as shown below.) But during those years, there was no knowledge about the atomic structure.
What is the order of the elements in the periodic table?
the elements are listed in order of increasing atomic number . The atomic number is the number of protons in the nucleus of an atom.In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column.
Why are arranged symlinks arranged?
They are arranged to highlight similarites between differnt elements.