See more
Who is Dmitri Mendeleev and what was his contribution to chemistry?
Dmitri Mendeleev was a Russian chemist. He discovered and created the periodic table of elements. He also devised the Periodic Law.
What was wrong with Mendeleev's periodic table?
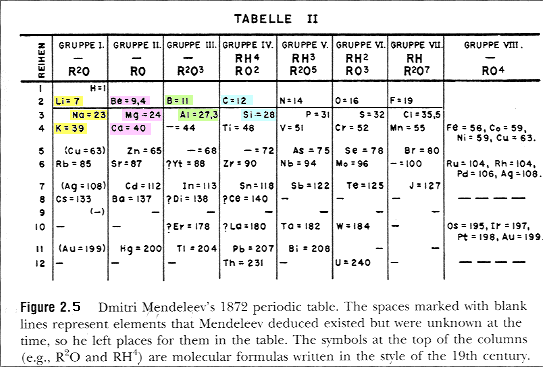
Mendeleev's periodic table was not accepted by everyone. This was because Mendeleev did not place all elements in ascending atomic mass order. Some...
How did Mendeleev arrange the periodic table?
Mendeleev arranged the periodic table by elements' atomic masses. The atomic mass increased as more elements were added in ascending order.
How is Mendeleev's periodic table different?
Mendeleev's periodic table is different because it left gaps for unknown elements at the time. Also, his periodic table did not contain the noble g...
What did Dmitri Mendeleev accomplish?
Dmitri Mendeleev devised the periodic classification of the chemical elements, in which the elements were arranged in order of increasing atomic we...
What was Dmitri Mendeleev’s early life like?
Dmitri Mendeleev’s parents were Ivan Mendeleev, a teacher, and Mariya Kornileva. Ivan went blind in 1834, the year Dmitri was born, and died in 184...
What was Dmitri Mendeleev’s occupation?
In 1865 Dmitri Mendeleev became professor of chemical technology at the University of St. Petersburg. He became professor of general chemistry ther...
Who Was Dmitri Mendeleev?
A chemist by both education and trade, Dmitri Mendeleev helped to transform the world of science with his way of organizing the elements. Mendeleev was born in Russia, where he spent much of his scientific career teaching and studying chemistry.
Why did Mendeleev think there were empty spaces in the periodic table?
While constructing his version of the periodic table, Mendeleev noticed that some spaces were better left empty, because there was no known element that had the necessary characteristics. Mendeleev hypothesized that these empty spaces were for elements that had not been discovered yet. So certain that he was right, Mendeleev went ahead and predicted the atomic weights and properties for these undiscovered elements. He even predicted the properties of germanium based on what he knew about the elements that lay above it in the periodic table. He called it 'eka-silicon,' using the sanskrit word eka (which means 'beyond') to suggest that it had properties similar to silicon and that it would be placed in the row below silicon.
How did Mendeleev organize elements?
Using this information and alleged inspiration from the card game solitaire, Mendeleev began grouping elements into rows (known as periods) and columns (known as groups ). He arranged elements in rows by increasing atomic weight, then linked those rows into columns based on similar properties.
Why did Mendeleev think that some spaces were better left empty?
While constructing his version of the periodic table, Mendeleev noticed that some spaces were better left empty, because there was no known element that had the necessary characteristics . Mendeleev hypothesized that these empty spaces were for elements that had not been discovered yet.
What is the significance of the periodic table?
The periodic table is one of the most recognized objects in modern society. Its design allows for easy grouping and identification of the known elements. This design, however, took time to develop.
Where did Dmitri Mendeleev go to university?
The Mendeleevs were a relatively well-off family who valued education. When Dmitri's father passed away in 1847, his mother took it upon herself to ensure that Dmitri went to university, taking him over 1,300 miles to St. Petersburg to enroll. Legend has it that Mrs. Mendeleev made the journey with Dimitri on horseback, then died shortly after he was accepted to the Institute of Pedagogy in St. Petersburg.
What does it mean to enroll in a course?
Enrolling in a course lets you earn progress by passing quizzes and exams.
What did Dmitri Mendeleev do?
What did Dmitri Mendeleev accomplish? Dmitri Mendeleev devised the periodic classification of the chemical elements, in which the elements were arranged in order of increasing atomic weight.
What did Mendeleev find?
Mendeleev found that, when all the known chemical elements were arranged in order of increasing atomic weight, the resulting table displayed a recurring pattern, or periodicity, of properties within groups of elements. In his version of the periodic table of 1871, he left gaps in places where he believed unknown elements would find their place.
What did Mendeleev do to teach inorganic chemistry?
Formulation of the periodic law. As he began to teach inorganic chemistry, Mendeleev could not find a textbook that met his needs. Since he had already published a textbook on organic chemistry in 1861 that had been awarded the prestigious Demidov Prize, he set out to write another one.
Where was Mendeleev born?
Mendeleev was born in the small Siberian town of Tobolsk as the last of 14 surviving children (or 13, depending on the source) of Ivan Pavlovich Mendeleev, a teacher at the local gymnasium, and Mariya Dmitriyevna Kornileva. Dmitri’s father became blind in the year of Dmitri’s birth and died in 1847. To support the family, his mother turned to operating a small glass factory owned by her family in a nearby town. The factory burned down in December 1848, and Dmitri’s mother took him to St. Petersburg, where he enrolled in the Main Pedagogical Institute. His mother died soon after, and Mendeleev graduated in 1855. He got his first teaching position at Simferopol in Crimea. He stayed there only two months and, after a short time at the lyceum of Odessa, decided to go back to St. Petersburg to continue his education. He received a master’s degree in 1856 and began to conduct research in organic chemistry. Financed by a government fellowship, he went to study abroad for two years at the University of Heidelberg. Instead of working closely with the prominent chemists of the university, including Robert Bunsen, Emil Erlenmeyer, and August Kekulé, he set up a laboratory in his own apartment. In September 1860 he attended the International Chemistry Congress in Karlsruhe, convened to discuss such crucial issues as atomic weights, chemical symbols, and chemical formulas. There he met and established contacts with many of Europe’s leading chemists. In later years Mendeleev would especially remember a paper circulated by the Italian chemist Stanislao Cannizzaro that clarified the notion of atomic weights.
Who discovered the periodic law?
Thus, in his effort to make sense of the extensive knowledge that already existed of the chemical and physical properties of the chemical elements and their compounds, Mendeleev discovered the periodic law. Mendeleev's periodic table. The periodic table of the elements from Dmitri Mendeleev's Osnovy khimii (1869; The Principles of Chemistry ), ...
Which element did Mendeleev compare to the properties of the group of alkali metals?
When Mendeleev began to compose the chapter on the halogen elements ( chlorine and its analogs) at the end of the first volume, he compared the properties of this group of elements to those of the group of alkali metals such as sodium.
When did Dmitri go blind?
Ivan went blind in 1834, the year Dmitri was born, and died in 1847. Mariya then ran a glass factory. However, the factory burned down in 1848, and Dmitri moved to St. Petersburg to continue his education.
Why did Mendeleev leave gaps in his table?
Predictions using gaps. Mendeleev left gaps in his table to place elements not known at the time. By looking at the chemical properties and physical properties of the elements next to a gap, he could also predict the properties of these undiscovered elements.
How did Mendeleev arrange the elements?
He then arranged the elements by putting those with similar properties below each other into groups. To make his classification work Mendeleev made a few changes to his order:
Which element was found to be similar to the predicted ones?
For example, Mendeleev predicted the existence of 'eka-silicon', which would fit into a gap next to silicon. The element germanium was discovered later. Its properties were found to be similar to the predicted ones and confirmed Mendeleev's periodic table.
Is iodine a chemical?
So iodine should be placed before tellurium in Mendeleev's tables. However, iodine has similar chemical properties to chlorine and bromine. To make iodine line up with chlorine and bromine in his table, Mendeleev swapped the positions of iodine and tellurium.
Who made the periodic table?
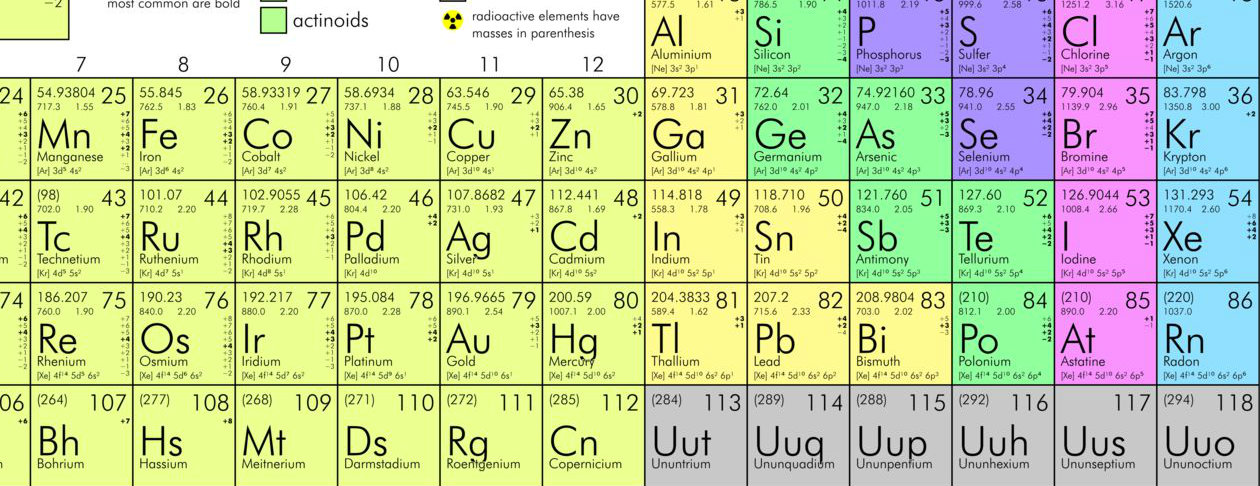
Mendeleev made an early periodic table. In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic configurations model how electrons are arranged in atoms.
Who was the Russian chemist who published the periodic table?
Dmitri Mendeleev. Like many scientists working at the end of the 19th-century the Russian chemist Dmitri Mendeleev (1834-1907) was looking for ways to organise the known elements. Mendeleev published his first periodic table of the elements in 1869.
What did Mendeleyev notice?
As Mendeleyev’s eyes ran once more along the line of ascending atomic weights, he suddenly noticed something that quickened his pulse. Certain similar properties seemed to repeat in the elements, at what appeared to be regular numerical intervals. Here was something! But what? A few of the intervals began with a certain regularity, but then the pattern just seemed to peter out. Despite this, Mendeleyev soon became convinced that he was on the brink of a major breakthrough. There was a definite pattern there somewhere, but he just couldn’t quite grasp it… Momentarily overcome by exhaustion, Mendeleyev leaned forward, resting his shaggy head on his arms. Almost immediately he fell asleep, and had a dream.
How many elements did Mendeleev classify?
By his mid-thirties, he was intensely preoccupied with classifying the 56 elements known by that point. He struggled to find an underlying principle that would organize them according to sets of similar properties and eventually reaped the benefits of the pattern-recognition that fuels creativity.
Who created the periodic table?
Trailblazing chemist Dmitri Mendeleev (February 8, 1834–February 2, 1907) came to scientific greatness via an unlikely path, overcoming towering odds to create the periodic table foundational to our understanding of chemistry. Born in Siberia as one of anywhere between 11 and 17 children — biographical accounts differ, as infant mortality rate in the era was devastatingly high — he was immersed in tragedy from an early age. His father was a professor of fine arts, philosophy, and politics, but grew blind and lost his teaching position. His mother became the sole breadwinner, working at a glass factory. When Dmitri was thirteen, his father died. Two years later, a fire destroyed the glass factory.
Who wrote Mendeleyev's dream?
In Mendeleyev’s Dream: The Quest for the Elements ( public library ), novelist Paul Strathern reconstructs the landmark moment from the scientist’s letters and diaries, and reimagines it with a dose of satisfying literary flourishing:
Why did Mendeleev put nickel and cobalt together?
But Mendeleev placed Cobalt (Co) along with Rhodium (Rh) because Cobalt (Co) and Rhodium (Rh) were having similar properties. Also the properties of Nickel (Ni) is similar to the Palladium element (Pd), so he placed Nickel (Ni) along with Palladium (Pd). Tellurium (Te) and Iodine (I) also show the exceptions.
What did Mendeleev find?
Now during the year 1869, Mendeleev was finding the relation between atomic mass of elements and their physical and chemical properties. Mendeleev found the chemical properties of known elements through their chemical reaction with hydrogen and oxygen. But the question is;
How many elements were in Mendeleev's first periodic table?
The elements in this First Periodic table were arranged in the increasing order of their atomic masses, and there were only 63 elements in his table. The genius work which Mendeleev did was to leave empty gaps for ...
Why did Mendeleev place some elements with higher atomic mass before lower ones?
The simple answer: Mendeleev placed some elements with higher atomic mass before lower ones, so that he can group the elements with similar properties together.
When was the periodic table first created?
And if the property of elements didn’t match, then he placed it in the row. In this way, Mendeleev prepared his first Periodic table in 1869.
When was Mendeleev's periodic table created?
In this way, Mendeleev prepared his first Periodic table in 1869. The modified version of Mendeleev’s Periodic table is as shown below. The important thing to note down here is that the elements in the above table are arranged in the increasing order of their Atomic Masses. The vertical columns are called the groups and ...
Which element is also known as the father of the periodic table?
Eka-silicon —> Germanium. And the main thing is that the properties of these three elements were almost similar to the properties that Mendeleev had already predicted. This was his greatest achievement. That is why Mendeleev is also known as Father of Periodic table.
