
See more
Why did Mendeleev leave gaps in his table?
Predictions using gaps. Mendeleev left gaps in his table to place elements not known at the time. By looking at the chemical properties and physical properties of the elements next to a gap, he could also predict the properties of these undiscovered elements.
How did Mendeleev arrange the elements?
He then arranged the elements by putting those with similar properties below each other into groups. To make his classification work Mendeleev made a few changes to his order:
Which element was found to be similar to the predicted ones?
For example, Mendeleev predicted the existence of 'eka-silicon', which would fit into a gap next to silicon. The element germanium was discovered later. Its properties were found to be similar to the predicted ones and confirmed Mendeleev's periodic table.
Is iodine a chemical?
So iodine should be placed before tellurium in Mendeleev's tables. However, iodine has similar chemical properties to chlorine and bromine. To make iodine line up with chlorine and bromine in his table, Mendeleev swapped the positions of iodine and tellurium.
Who made the periodic table?
Mendeleev made an early periodic table. In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic configurations model how electrons are arranged in atoms.
Who was the Russian chemist who published the periodic table?
Dmitri Mendeleev. Like many scientists working at the end of the 19th-century the Russian chemist Dmitri Mendeleev (1834-1907) was looking for ways to organise the known elements. Mendeleev published his first periodic table of the elements in 1869.
What elements were discovered by Mendeleev?
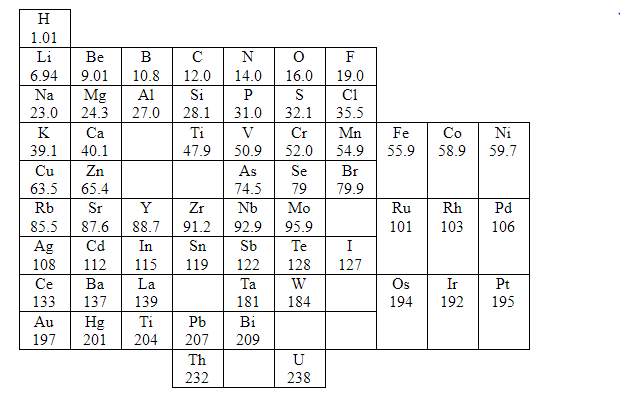
Some of these are scandium (Sc), gallium (Ga) and germanium (Ge). These elements were accommodated in their proper places in the table. The elements helium (He), neon (Ne), argon (Ar), Krypton (Kr), Xenon (Xe) and radon (Rn) became known only towards the end of the nineteenth century. These elements, called noble gases, were placed in the table as a separate group, called 0 group. The periodic table had to be modified then. The modified version of the table is shown below.
What are the properties of elements?
The properties of elements are periodic functions of their atomic masses. This means, if the elements are arranged in order of increasing atomic masses then those with similar properties are repeated at regular intervals. On the basis of the periodic law, Mendeleev presented his classification in the form of a table, ...
Why is titanium placed below silicon?
Considering its atomic mass, titanium (Ti) should have been placed below aluminium in the periodic table, but Mendeleev placed is below silicon (Si) because the properties of titanium were similar to those of silicon. Thus, a gap was left below aluminium in the periodic table. This gap was filled up by gallium which was discovered later. The properties of gallium (Ga) were found to be similar to those of boron and aluminium.
How many elements were found in Mendeleeve's periodic table?
He arranged the 63 elements then known in a table on the basis of similarities in properties. It was found that most of the elements occupied places in the table in order of their increasing atomic masses. In 1869, Mendeleeve formulated a law, now known as the periodic law. The law is stated as follows.
What are the defects in Mendeleev's periodic table?
Mendeleev’s periodic table has the following defects. 1. Position of hydrogen : The position of hydrogen in the periodic table is anomalous. Hydrogen resembles alkali metals (Li, Na, K, etc). So it may be placed in the group of the halogens (VII A) 2.
What was Mandeleev's idea of classification?
So, he did not provide any place for them in his periodic table. Mandeleev’s idea was remarkable in that he used a fundamental atomic property (atomic mass) as the basis of classification. While classifying elements he laid special emphasis on tow factors. 1. Similar elements were grouped together.
Which physicist allowed the subgroups to be represented within the same group?
Mendeleev allowed the subgroups to be represented within the same group.
How did Mendeleev organize elements?
Mendeleev arranged the elements in order of increasing relative atomic mass. When he did this he noted that the chemical properties of the elements and their compounds showed a periodic trend. He then arranged the elements by putting those with similar properties below each other into groups. To make his classification work Mendeleev made a few changes to his order: 1 he left gaps for yet to be discovered elements 2 he switched the order of a few elements to keep the groups consistent
Why did Mendeleev leave gaps in his table?
Mendeleev left gaps in his table to place elements not known at the time. By looking at the chemical properties and physical properties of the elements next to a gap, he could also predict the properties of these undiscovered elements.
Which element was found to be similar to the predicted ones?
For example, Mendeleev predicted the existence of 'eka-silicon', which would fit into a gap next to silicon. The element germanium was discovered later. Its properties were found to be similar to the predicted ones and confirmed Mendeleev's periodic table.
Is iodine a chemical?
So iodine should be placed before tellurium in Mendeleev's tables. However, iodine has similar chemical properties to chlorine and bromine. To make iodine line up with chlorine and bromine in his table, Mendeleev swapped the positions of iodine and tellurium.
Who made the periodic table?
Mendeleev made an early periodic table. In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic configurations model how electrons are arranged in atoms.
Who was the Russian chemist who published the periodic table?
Dmitri Mendeleev. Like many scientists working at the end of the 19th-century the Russian chemist Dmitri Mendeleev (1834-1907) was looking for ways to organise the known elements. Mendeleev published his first periodic table of the elements in 1869.
