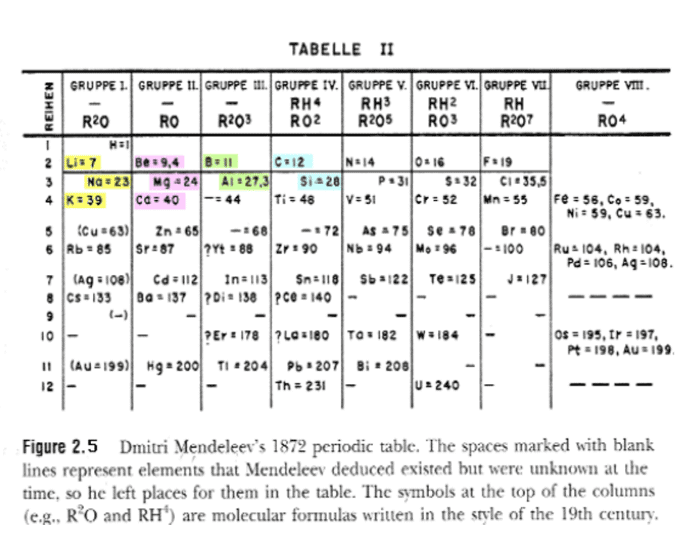
How did Mendeleev organize his periodic table of the elements?
Mendeleev arranged the elements in order of increasing relative atomic mass . When he did this he noted that the chemical properties of the elements and their compounds showed a periodic trend .
How was the periodic table organized?
How is the Periodic Table arranged? The periodic table is arranged by atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row (called a period) and column (called a group). The table comprises seven rows and 18 columns.
How did Mendeleev organize the periodic table of elements quizlet?
How did Mendeleev organize the elements in his periodic table? arranged the elements into rows in order of increasing mass so that elements with similar properties were in the same column.
How is the periodic table organized according to Mendeleev and Moseley?
The Mendeleev periodic table has chemical elements arranged based on their atomic masses. The Moseley periodic table has chemical elements arranged based on their atomic numbers.
How is the periodic table organized quizlet?
The periodic table is organized base on increasing atomic number. It is then organized into groups, or columns, and periods, or rows. Elements in the same groups have similar characteristics, and periods are based on increasing valence electrons, atomic mass, and atomic number.
How did Henry D Hubbard rearrange the elements?
His version of the periodic table placed the main groups in columns with some later groups taking up two rows per period and the Group VIIIB transition metals displayed out to the right of the noble gases. The noble gases themselves were shown first in Column 1 (Valence 0) and repeated in Column 9 (Group VIII).
How did Moseley establish a more accurate periodic table?
Answer and Explanation: Henry Moseley in 1913 proposed organizing Mendeleev's periodic table based on physical properties based on atomic number rather than atomic mass. When this was done, the organization of the table showed more consistent periodic patterns of the physical properties.
Who arranged the periodic table by atomic number?
Dmitri MendeleevAlbert GhiorsoPeriodic table/Inventors