
Is there an easy way to find number of valence electrons"?
To find valence electrons using a period table , first see if your atom is a transitional metal, which are the elements in the middle rectangle of the table. If the atom is outside this block, locate its group number along the top of the table. The ones digit in the group number is the number of valence electrons.
Which elements have 5 valence electrons?
Insulators
- Nitrogen has \ (5\) valence electrons
- Sulphur has \ (6\) valence electrons
- Neon has \ (8\) Valence electrons.
How do you calculate valence?
How do you calculate valence electron concentration? To calculate the valence-electron contribution of an element one has to subtract 8 from the total number of outer-shell electrons er which is equal to the group number of the transition element in the periodic system.For example Pd with er= 10 provides only 2 valence electrons.
Are valence electrons different than other electrons?
While all electrons play a role, none of the electrons play a bigger role than the valence electrons. Valence electrons are the electrons that are located in the outermost energy level of an atom. Some elements will have the same number of valence electrons in a neutral atom, but the number and arrangement of the electrons will be different.
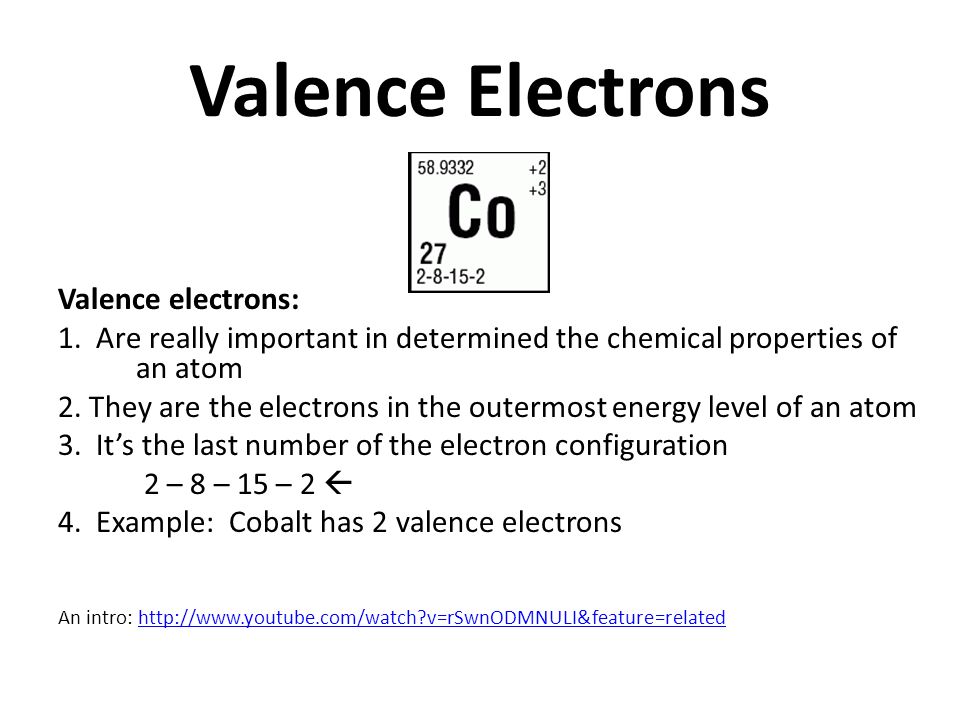
How do you find the valence electrons?
For neutral atoms, the number of valence electrons is equal to the atom’s main group number. The main group number for an element can be found from...
What is a valence electron with example?
The total number of electrons present in last shell orbit is known as valence electron. For example: Oxygen has 6 electrons present in last orbital...
What is called valency?
Valency is the combining power of an element. Elements in the same group of the periodic table have the same valency. The valency of an element is...
What is the purpose of valence electrons?
Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom.
How do valence electrons work?
Valence electrons are the electrons located at the outermost shell of an atom. … Because when two atoms interact, the electrons in the outermost sh...
How to find valence electrons in a period table?
To find valence electrons using a period table, first see if your atom is a transitional metal, which are the elements in the middle rectangle of the table. If the atom is outside this block, locate its group number along the top of the table. The ones digit in the group number is the number of valence electrons.
How to determine the number of valence electrons?
Determine the number of valence electrons based on the group number. Once again, the group number of the element you are examining can tell you its valence electrons. However, for the transition metals, there isn't a pattern you can follow — group number will usually correspond to a range of possible numbers of valence electrons. These are:
How to find the number of valence electrons in an atom?
Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones place of the group number is the number of valence electrons in an atom of these elements. In other words:
How many electrons does sodium have?
So, for our example, we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital. That's 11 electrons total — sodium is element number 11, so this makes sense.
How many valence electrons does carbon have?
In our example, since carbon is in group 14, we can say that one atom of carbon has four valence electrons.
How to label a column on the periodic table?
Label each column on the periodic table of elements from 1 to 18. Generally, on a periodic table, all of the elements in a single vertical column will have the same number of valence electrons. If your periodic table doesn't already have each column numbered, give each a number starting with 1 for the far left end and 18 for the far right end. In scientific terms, these columns are called the element "groups."
How many orbital shells does Selenium have?
For example, we know the element selenium has four orbital shells because it is in the fourth period. Since it is the sixth element from the left in the fourth period (ignoring the transition metals), we know that the outer fourth shell has six electrons, and, thus, that Selenium has six valence electrons.
Where are the valence electrons located?
Valence electrons are the electrons located at the outermost shell of an atom. … Because when two atoms interact, the electrons in the outermost shells are the first ones to come into contact with each other and are the ones that determine how an atom will react in a chemical reaction.
What are Valence Electrons?
Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. “Electrons in the outer shells that are not filled are called valence electrons”.
How many electrons are in the last shell of an orbit?
The total number of electrons present in last shell orbit is known as valence electron. For example: Oxygen have 6 electrons present in last orbital shell hence 6 is valence electron.
How stable are atoms?
Atoms are most stable if they have a filled valence shell of electrons. Atoms transfer or share electrons in such a way that they can attain a filled shell of electrons. For the main group elements, the valence electron exists only in the outermost electron shell. A valence electron can exist in the inner shell of a transition metal.
Why are electrons important in chemistry?
Electrons are involved in the chemical bonding and reactions of the atom. It is said to occupy orbitals in an atom. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Atoms are most stable if they have a filled valence shell of electrons.
What are the s and p electrons in the outermost shell?
Valence electrons are the s and p electrons in the outermost shell. The electrons present in the inner shell are core electrons. When we study and observe the atom of an element, we come across tiny subatomic particles called valence electrons.
Why are valence electrons important in chemical reactions?
The valence electrons are part of most of the chemical reactions because they contain more energy compared to the electrons present in inner orbits. Meanwhile, the number of valence electrons present also helps us determine a specific element’s chemical properties, such as its valence or valency, the formation of bonds with other elements. It also gives us an idea of how readily the atoms can form bonds, the number of unpaired electrons and how many atoms can take part.
