What trend in ionization energy occurs across a period on the periodic table?
Ionization energy exhibits periodicity on the periodic table. The general trend is for ionization energy to increase moving from left to right across an element period. Moving left to right across a period, atomic radius decreases, so electrons are more attracted to the (closer) nucleus.
What is the pattern for ionization energy in a period?
Ionization energy generally increases moving from left to right across an element period (row). This is because the atomic radius generally decreases moving across a period, so there is a greater effective attraction between the negatively charged electrons and positively-charged nucleus.
Does atomic size generally increase across a period?
This is caused by the increase in the number of protons and electrons across a period. One proton has a greater effect than one electron; thus, electrons are pulled towards the nucleus, resulting in a smaller radius. Atomic size INCREASES down a Group, but DECREASES across a Period.
Does energy always released during ionization?
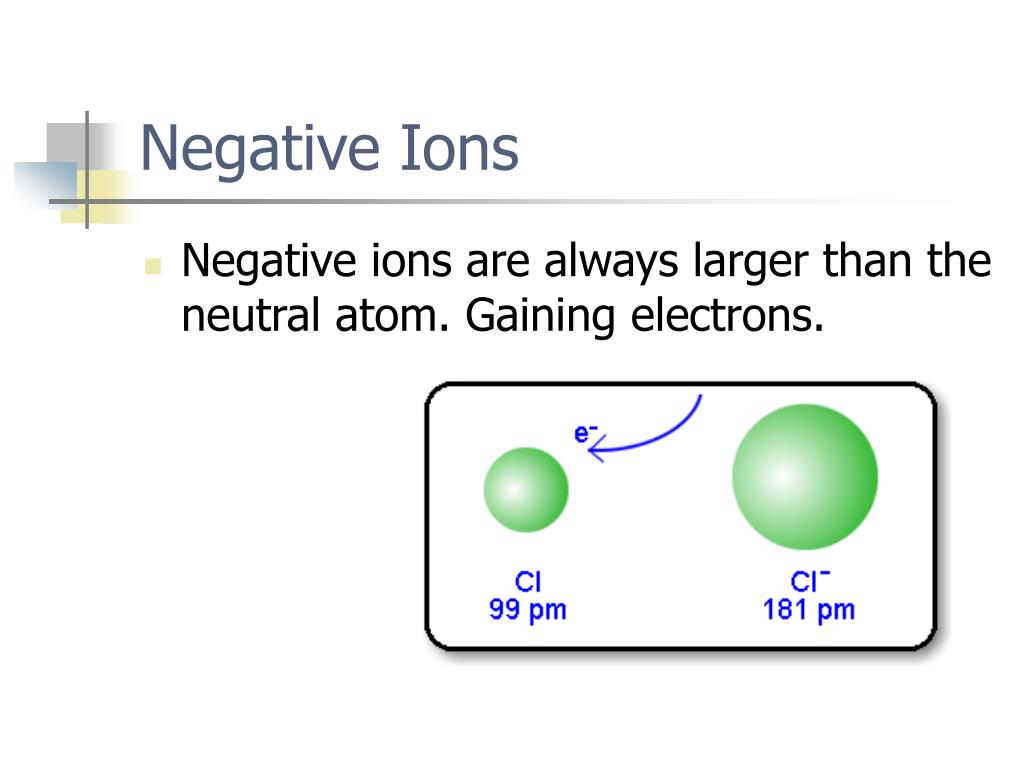
Ionization of Atoms. When an atom gains an electron, energy is usually released. This energy is called the electron affinity of that atomic species. Atoms that have a large electron affinity are more likely to gain an electron and form negative ions. Loss of an electron from an atom requires energy input.

Why does ionization energy change across a period?
On the periodic table, first ionization energy generally increases as you move left to right across a period. This is due to increasing nuclear charge, which results in the outermost electron being more strongly bound to the nucleus.
Why does ionization energy increase across a period and decrease down a group?
On the periodic table, first ionization energy generally decreases as you move down a group. This is because the outermost electron is, on average, farther from the nucleus, meaning it is held less tightly and requires less energy to remove.
Why does ionization energy decrease from left to right?
Since the outermost electrons are further away, they are less strongly attracted by the nucleus, and are easier to remove, corresponding to a lower value for the first ionization energy.
Why does ionization energy increase from bottom to top?
This is because the valence electrons do not screen each other very well, allowing the effective nuclear charge to increase steadily across the row. The valence electrons are therefore attracted more strongly to the nucleus, so atomic sizes decrease and ionization energies increase.
Why does ionisation energy decrease down a group?
As we move down the group, ionization energy decreases because while moving down the group atomic size increases due to this fact the force of attraction between the nucleus and the valence electrons is weaker. So, it becomes easy to remove an electron from an atom. Thus, the ionization decreases down the group.
Does ionization energy increase or decrease down a group?
Down a group, ionization energies decrease. This is because as you go down a group, electrons are located in successively higher energy levels, farther away from the attraction of the nucleus. Furthermore, down a group, there are more electrons between the outside valence electrons and the nucleus.
What is ionization energy?
Ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. The most common units of ionization energy are kilojoules per mole (kJ/M) or electron volts (eV). Ionization energy exhibits periodicity on the periodic table. The general trend is for ionization energy to increase moving from left ...
What is the trend for ionization energy to decrease moving from top to bottom down a periodic table group?
The general trend is for ionization energy to decrease moving from top to bottom down a periodic table group. Moving down a group, a valence shell is added. The outermost electrons are further from the positive-charged nucleus, so they are easier to remove.
Why does ionization decrease as electrons move down the group?
This is because the principal quantum number of the outermost electron increases moving down a group. There are more protons in atoms moving down a group (greater positive charge), yet the effect is to pull in the electron shells, making them smaller and screening outer electrons from the attractive force of the nucleus. More electron shells are added moving down a group, so the outermost electron becomes increasingly distance from the nucleus.
What are the exceptions to the ionization energy trend?
Exceptions to the Ionization Energy Trend. If you look at a chart of first ionization energies, two exceptions to the trend are readily apparent. The first ionization energy of boron is less than that of beryllium and the first ionization energy of oxygen is less than that of nitrogen.
Why is ionization energy important?
Ionization energy is important because it can be used to help predict the strength of chemical bonds. Also Known As: ionization potential, IE, IP, ΔH°. Units: Ionization energy is reported in units of kilojoule per mole (kJ/mol) or electron volts (eV).
What happens to ionization energy when moving from left to right?
Moving left to right across a period, atomic radius decreases, so electrons are more attracted to the (closer) nucleus.
What is the trend of ionization?
Ionization, together with atomic and ionic radius, electronegativity, electron affinity, and metallicity, follows a trend on the periodic table of elements. Ionization energy generally increases moving from left to right across an element period ( row). This is because the atomic radius generally decreases moving across a period, ...
Why does ionization energy decrease as you move down?
However, ionization energy will decrease as you move down (through groups) the PToE because of something we call electron shielding. This has two main factors, 1. the valence electrons are farther from the protons keeping them with the atom, and 2. the electrons from the lower shells are also pushing the upper ones away.
What happens to ionization energy as you move?
As you move across a period, first ionization energy increases.
How do electrons affect the periodic table?
When considering the periodic trends you should evaluate the effect of three main factors. 1. The effective nuclear charge- which is roughly equal to the number of protons minus the number of shielding electrons, 2. The shielding of the valence electrons by the core electrons and 3. The electron electron repulsions that result when more electrons occupy the same energy level or sublevel . As you move across a period on the periodic table the increase in the number of protons attracts electrons more. This is while the number of core electrons remains the same. The result is a higher energy requirement to remove an electron on the right side of the table compared to an electron on the left side. There are some exceptions to this general trend. Notably nitrogen and oxygen. Oxygen’s valence electrons experience a higher nuclear charge compared to nitrogen’s but oxygen’s valence sub level of 2p contains an addition electron. In this instance the extra repulsion from that addition electron outweighs the added proton. The result is a lower ionization energy. In another exception, Boron, which has an additional proton compared to beryllium still has a lower ionization energy because the boron atom has an electron in a 2p orbital - this electron experiences partial shielding by its 2s electron. Be, despite having one less proton has a 2s electron that is only shielded by its 1s electrons.
How do periodic trends affect the electron repulsions?
The effective nuclear charge- which is roughly equal to the number of protons minus the number of shielding electrons, 2. The shielding of the valence electrons by the core electrons and 3. The electron electron repulsions that result when more electrons occupy the same energy level or sublevel . As you move across a period on the periodic table the increase in the number of protons attracts electrons more. This is while the number of core electrons remains the same. The result is a higher energy requ
What happens to the ionization energy of the representative elements in a period when moving from left to right?
Thus, on moving from left to right in a period, the tendency of atoms to lose electrons decreases. Hence, the ionization energy increases across the period. In general, the first ionization energy of the representative elements in a period increases from left to right.
Why is it harder to remove the outermost electron?
WHY? - As you move across a period, the atomic radius decreases, that is, the atom is smaller. The outer electrons are closer to the nucleus and more strongly attracted to the center. Therefore, it becomes more difficult to remove the outermost electron.
How to determine the size of an atom?
When we consider the size of an atom, there are three factors that we need to consider: 1 The amount of energy levels filled. In hydrogen the second energy level has an average radius four times as large as the first, the third nine times, the fourth 16 times and so on and so forth. Although this does not mean that between periods the size grows in this way (you also need to take into account the nucleus charge), this is still the most important factor. An increase in period leads to a significant increase in atom size and a decrease to a significant decrease. 2 The nucleus charge: the larger the am
