What are facts about the periodic table?
Fun facts about the Periodic Table
- Carbon is unique in that it is known to form up to 10 million different compounds. ...
- Francium is the rarest element on earth. ...
- The only letter not in the periodic table is the letter J.
- The country Argentina is named after the element silver (symbol Ag) which is argentum in Latin.
What are the basics of the periodic table?
These properties and their trends are:
- Ionization Energy - energy needed to remove an electron from a gaseous atom or ion. ...
- Electronegativity - how likely an atom is to form a chemical bond. ...
- Atomic Radius (and Ionic Radius) - a measure of the size of an atom. ...
- Electron Affinity - how readily an atom accepts an electron. ...
What is the periodic table used for?
The periodic table, also known as the periodic table of ( the) ( chemical) elements, is a tabular display of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry.
How do you understand the periodic table?
Vocabulary
- Elements: Substances consisting of only one atom.
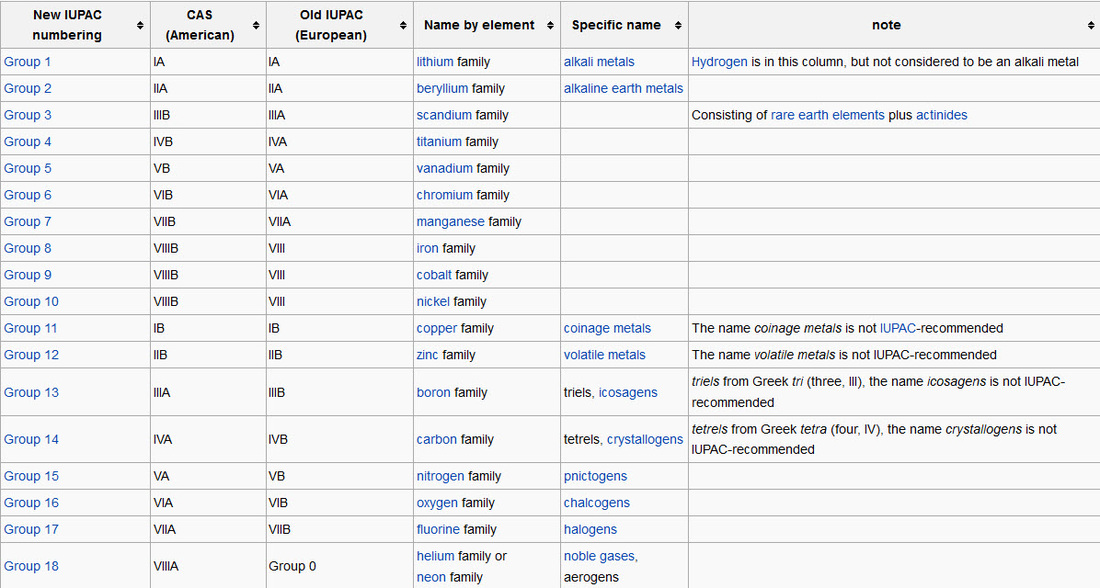
- Groups: The vertical column of the periodic table signifies the number of valence electrons in an element.
- Periods: The horizontal rows in a periodic table indicate the number of electron shells in an element.
How does an element on the periodic table work?
The periodic table is a graphical collection of element data. The table lists the chemical elements in order of increasing atomic number, which is the number of protons in an atom of an element. The rows (periods) and columns (groups) organize elements according to similar properties.
How do you read the periodic table?
When you're reading the periodic table, move across the table from top left to bottom right. As you move across the table, the number of protons and the atomic mass of each element increases. Each element has its own atomic number, which represents the number of protons in one atom of the element.
How is the periodic table arranged?
The periodic table is arranged by atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row (called a period) and column (called a group). The table comprises seven rows and 18 columns.
How do you explain the periodic table to a child?
The periodic table is a system for arranging the chemical elements. The chemical elements are the basic substances that make up all matter. Each chemical element has a particular feature called its atomic number. That number comes from the amount of tiny particles called protons in each atom of the element.
What is the easiest way to learn periodic table?
Memorization StrategiesBreak down the table into sections. ... Spread out the memorization process. ... Learn the elements in a song. ... Make nonsense words made from element symbols. ... Use color to learn element groups. ... Use a mnemonic device to help remember the order of the elements.
How can I memorize the periodic table?
You could use the acronym HHeLiBeBCNOF (pronounced 'heeliebeb kernoff') to remember the first nine chemical elements. It's a nonsense word, but it condenses nine names into one mental prompt or cue. Or the acrostic “Here He Lies Beneath Bed Clothes, Nothing On, Feeling Nervous” would equate to H He Li Be B C N O F Ne.
What are 118 elements called?
The permanent names for elements 113, 115, 117, and 118 are nihonium, moscovium, tennessine, and oganesson.
What are the 3 ways the periodic table is organized?
Here's how it works:Elements are listed in numerical order by atomic number. ... Each horizontal row on the periodic table is called a period. ... Each vertical column on the periodic table is called a group. ... There are two rows of elements found below the main body of the periodic table.More items...•
Why is the periodic table shaped that way?
Why does the periodic table have the structure it does? The answer is rather simple, if you understand electron configurations: the shape of the periodic table mimics the filling of the subshells with electrons. The shape of the periodic table mimics the filling of the subshells with electrons.
What is the periodic table in simple terms?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson.
What are 5 facts about the periodic table?
15 Fun and Surprising Facts About the Periodic Table of ElementsDmitri Mendeleyev is the inventor of the modern periodic table. ... Scientists used battery polarity to weigh the elements. ... The periodic table reflects its creator's love for card games. ... It was used to correctly predict elements that hadn't been discovered.More items...•
How do I teach my 5 year old the periodic table?
5 Fun Ways to Teach the Periodic TableTry a merka Learning Kit. ... Go Over It Each Day (in Song) ... Play an Educational App. ... Use Fun Fact Flash Cards. ... Build Your Own with Post-It Notes.
How do you read groups and periods on a periodic table?
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
How do you read atomic symbols?
The symbol for an atom indicates the element via its usual two-letter symbol, the mass number as a left superscript, the atomic number as a left subscript (sometimes omitted), and the charge as a right superscript.
What does the period number tell you?
The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons.
How do you read the atomic number?
0:072:23Understanding Atomic Number and Atomic Mass - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe number that appears below the element symbol is called the atomic mass the mass of an atomMoreThe number that appears below the element symbol is called the atomic mass the mass of an atom depends on the number of protons neutrons.
What is the periodic table?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen...
What do periodic table groups have in common?
The groups of the periodic table are displayed as vertical columns numbered from 1 to 18. The elements in a group have very similar chemical proper...
Where does the periodic table come from?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion princ...
Why does the periodic table split?
The periodic table has two rows at the bottom that are usually split out from the main body of the table. These rows contain elements in the lantha...
Why is the periodic table organized?
The periodic table is organized to showcase the different trends (periodicity).
What is periodic table?
The periodic table is a graphical collection of element data. The table lists the chemical elements in order of increasing atomic number, which is the number of protons in an atom of an element. The rows (periods) and columns (groups) organize elements according to similar properties. For example, all of the elements in ...
What is the atomic number of an element?
The atomic number is how many protons an atom of that element contains. The number of protons is the deciding factor when distinguishing one element from another. Variation in the number of electrons or neutrons does not change the type of element.
What are the vertical columns in the periodic table called?
Mendeleev's original periodic table organized elements in order of increasing atomic mass or weight. The vertical columns are called groups. Each element in a group has the same number of valence electrons and typically behave in a similar manner when bonding with other elements. The horizontal rows are called periods.
Why is the periodic table given a range?
When a range is given, it's because the abundance of isotopes varies from one sampling location to another.
What happens when the number of electrons changes?
Changing number of electrons produces ions while changing the number of neutrons produces isotopes . The element's atomic mass in atomic mass units is a weighted average mass of the element's isotopes. Sometimes a periodic table cites a single value for atomic weight.
What is the valence of all elements in the first column?
For example, all of the elements in the first column are reactive metals that have a valence of +1. All elements in a row have the same outermost electron shell. A good periodic table is a great tool for solving chemistry problems. You can use an online periodic table or print your own.
How are periodic tables arranged?
The table is arranged in three main ways: by group, period and block. The table’s groups are the 18 vertical columns that run from left to right. Groups are considered the most important classification method, as they stack elements that have very similar properties and trend well throughout. The table’s periods are the top seven horizontal rows on the grid running top to bottom, and partner elements across the groups that share similarities in their properties.
Why are groups important in classification?
Groups are considered the most important classification method, as they stack elements that have very similar properties and trend well throughout. The table’s periods are the top seven horizontal rows on the grid running top to bottom, and partner elements across the groups that share similarities in their properties.
What is the periodic table?
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, ...
Why do the elements in the periodic table have different orbits?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion principle, no more than two electrons can fill the same orbital. The first row of the periodic table consists of just two elements, hydrogen and helium. As atoms have more electrons, they have more orbits available to fill, and thus the rows contain more elements farther down in the table.
What is the atomic number of an element?
The atomic number of an element is the number of protons in the nucleus of an atom of that element . Hydrogen has 1 proton, and oganesson has ...
What elements are triads?
Döbereiner in 1817 showed that the combining weight, meaning atomic weight, of strontium lies midway between those of calcium and barium, and some years later he showed that other such “ triads ” exist (chlorine, bromine, and iodine [halogens] and lithium, sodium, and potassium [alkali metals]). J.-B.-A. Dumas, L. Gmelin, E. Lenssen, Max von Pettenkofer, and J.P. Cooke expanded Döbereiner’s suggestions between 1827 and 1858 by showing that similar relationships extended further than the triads of elements, fluorine being added to the halogens and magnesium to the alkaline-earth metals, while oxygen, sulfur, selenium, and tellurium were classed as one family and nitrogen, phosphorus, arsenic, antimony, and bismuth as another family of elements.
Who proposed the periodic law?
Then in 1869, as a result of an extensive correlation of the properties and the atomic weights of the elements, with special attention to valency (that is, the number of single bonds the element can form), Mendeleyev proposed the periodic law, by which “the elements arranged according to the magnitude of atomic weights show a periodic change of properties.” Lothar Meyer had independently reached a similar conclusion, published after the appearance of Mendeleyev ’s paper.
Who proposed the atomic weights of the elements?
Attempts were later made to show that the atomic weights of the elements could be expressed by an arithmetic function, and in 1862 A.-E.-B. de Chancourtois proposed a classification of the elements based on the new values of atomic weights given by Stanislao Cannizzaro’s system of 1858.
When the chemical elements are thus arranged, there is a recurring pattern called the periodic law?
When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. The initial discovery, which was made by Dmitry I. Mendeleyev in the mid-19th century, has been of inestimable value in the development of chemistry.
What is the periodic table?
First, a quick review of what the periodic table is. It’s a chart of all the chemical building blocks of matter. To date, humans have observed 118, both natural and artificially made. Each of these building blocks, known as atomic elements, contains a positively charged core (known as the nucleus) that is ...
What atoms are used in clocks?
More recently, NIST researchers are making clocks with other atoms such as strontium, ytterbium, mercury and aluminum. The researchers change the quantum states of these atoms using optical radiation, with frequencies of hundreds of trillions of cycles per second (much higher than the microwave radiation used in cesium clocks).
Why are atomic clocks useful?
Precise time measurements are useful for time-stamping financial transactions, synchronizing communications and data, and navigating using the Global Positioning System (GPS). More recently, NIST researchers are making clocks with other atoms such as strontium, ytterbium, mercury and aluminum. The researchers change the quantum states of these atoms using optical radiation, with frequencies of hundreds of trillions of cycles per second (much higher than the microwave radiation used in cesium clocks). These “optical clocks” enable the second to be split into even smaller intervals that could be useful for things such as detecting underground geologic deposits and even dark matter.
What is the blue light on the periodic table?
A blue laser beam excites a cube-shaped cloud of strontium atoms located behind the round window in the middle of the table. Strontium atoms fluorescence strongly when excited with blue light. If you love numbers, well, of course, the periodic table is filled with them. Each atom on the table has a bevy of quantities.
What element did Mendeleev predict?
The genius of Mendeleev was that he left spaces for elements yet to be discovered, and in so doing he predicted their existence, such as gallium in 1875 and germanium in 1886. As you may have guessed, the latter was named after Germany (the home country of discoverer Clemens Winkler). As for the former, Paul Emile Lecoq de Boisbaudran named the element “gallia,” after Gaul, the Iron Age region that includes present-day France.
What are the properties of atoms in the same column?
For example, atoms in the rightmost column, known as the noble gases, may differ greatly in mass from light (helium) to heavy ( such as radon), but what they have in common is that they don’t ordinarily participate in chemical reactions.
What is the purpose of deuterium isotopes?
The deuterium isotope helps create heavier elements inside stars, makes certain drugs more effective, and could be the key ingredient for making clean fusion energy. It was discovered in the 1930s at the National Institute of Standards and Technology (NIST, then known as the National Bureau of Standards), where it was identified by Harold Urey of Columbia University, who won a Nobel Prize for the feat.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
What is PubChem working with?
PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
How does the periodic table organize the elements?
The classic Periodic Table organizes the chemical elements according to the number of protons that each has in its atomic nucleus. (Image credit: Karl Tate, Livescience.com contributor)
What do the columns of a table represent?
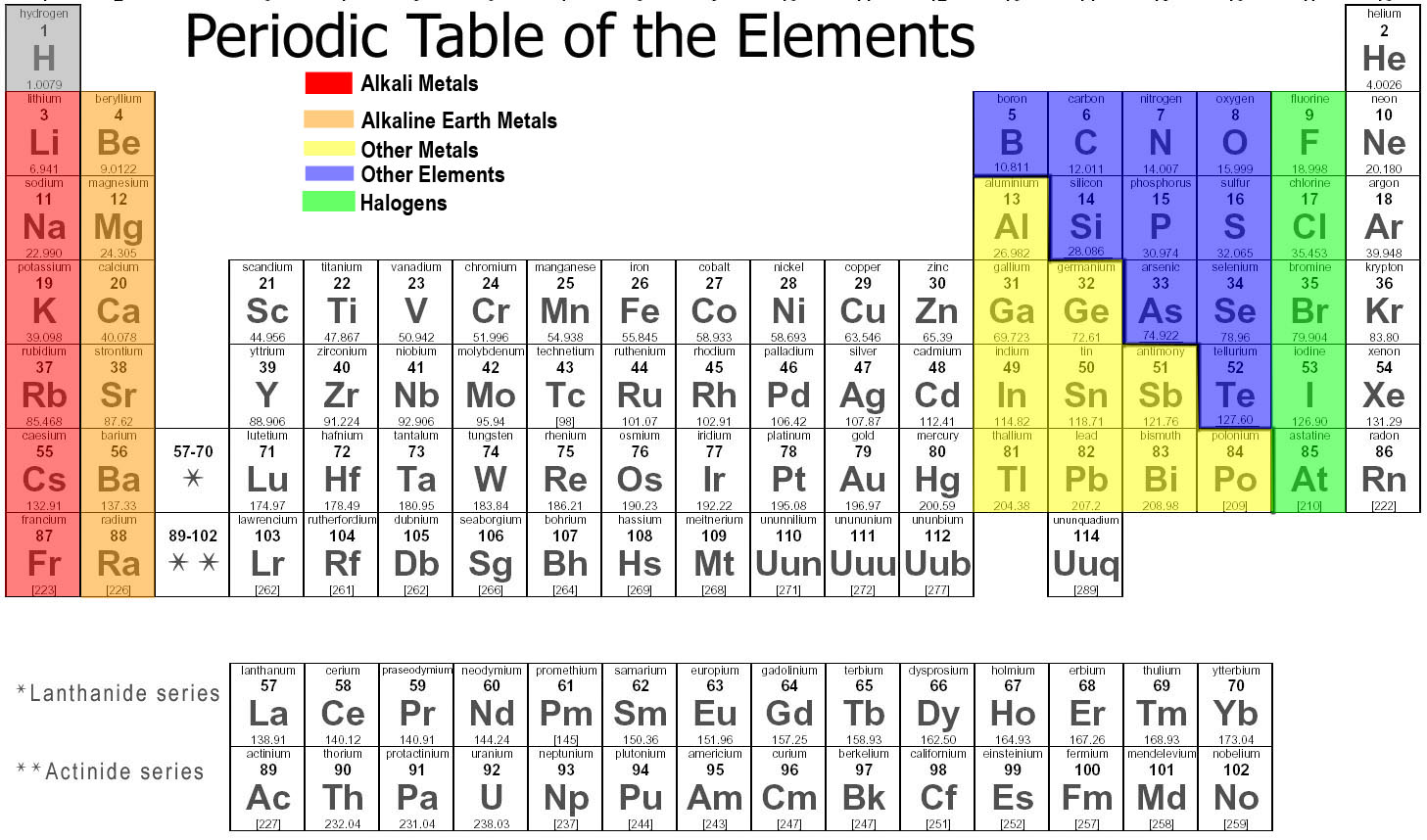
The columns of the table represent groups, or families, of elements. The elements in a group often look and behave similarly, because they have the same number of electrons in their outermost shell — the face they show to the world. Group 18 elements, on the far right side of the table, for example, have completely full outer shells ...
What are the groups of metals that are radioactive?
All are radioactive. The actinides and the lanthanides together form a group called the inner transition metals. Transition metals: Returning to the main body of the table, the remainder of Groups 3 through 12 represent the rest of the transition metals.
What are the elements in the actinides?
Actinides: The actinides line the bottom row of the island and comprise elements 89, actinium (Ac), through 103, lawrencium (Lr). Of these elements, only thorium (Th) and uranium (U) occur naturally on Earth in substantial amounts. All are radioactive. The actinides and the lanthanides together form a group called the inner transition metals.
What is table salt?
The table salt in your kitchen, for example, is a marriage between the alkali metal sodium and the halogen chlorine. Noble gases: Colorless, odorless and almost completely nonreactive, the inert, or noble gases round out the table in Group 18.
How many elements were there at the time of Mendeleev?
There were only about 60 elements known at the time, but Mendeleev realized that when the elements were organized by weight, certain types of elements occurred in regular intervals, or periods. Today, 150 years later, chemists officially recognize 118 elements (after the addition of four newcomers in 2016) and still use Mendeleev's periodic table ...
Why is the period of sodium longer?
Moving down the table, periods are longer because it takes more electrons to fill the larger and more complex outer levels. The columns of the table represent groups, or families, of elements.
How to read the periodic table?
To read the periodic table, start at the top left with the elements with the lowest atomic numbers, which tells you how many protons each atom has. Then, as you move right across the chart, make note that the atomic weight, shown at the bottom of the square, also increases.
Why does the periodic table have gaps?
Since elements don’t always fall neatly into groupings as they increase in number, the periodic table contains gaps. For example, the first 3 rows have gaps, as the Transition Metals don’t appear on the table until atomic number 21.
Why does the atomic mass increase as you move down the table?
The atomic mass increases as you move across or down the table because the mass is calculated by adding up the protons and neutrons in each element’s atom. The number of protons increases with each element, which means the weight goes up, as well.
How are elements ordered?
The elements are ordered by their atomic numbers, which increase as you move across and down the periodic table. The atomic number is how many protons the element’s atom possesses. You’ll also notice that each element’s atomic mass increases as you move across the table.
How does wikihow mark an article as reader approved?
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 85% of readers who voted found the article helpful, earning it our reader-approved status.
Why do most elements have atomic weights that include decimals?
Because the weights are averaged, most elements will have atomic weights that include decimals.
What are the gaps in the table?
Since the elements are also arranged by group, you will see gaps on the table. For example, the first row contains Hydrogen, which has an atomic number of 1, and Helium, which has an atomic number of 2. However, they are at opposite ends of the table, as they are in different groups.
