How many elements can you find on a periodic table?
Unless you currently work in a field that involves science, the days of memorizing the elements of the period table are long behind you. There are currently 118 known elements on the periodic table, and we’ve put 20 of them below to test your smarts.
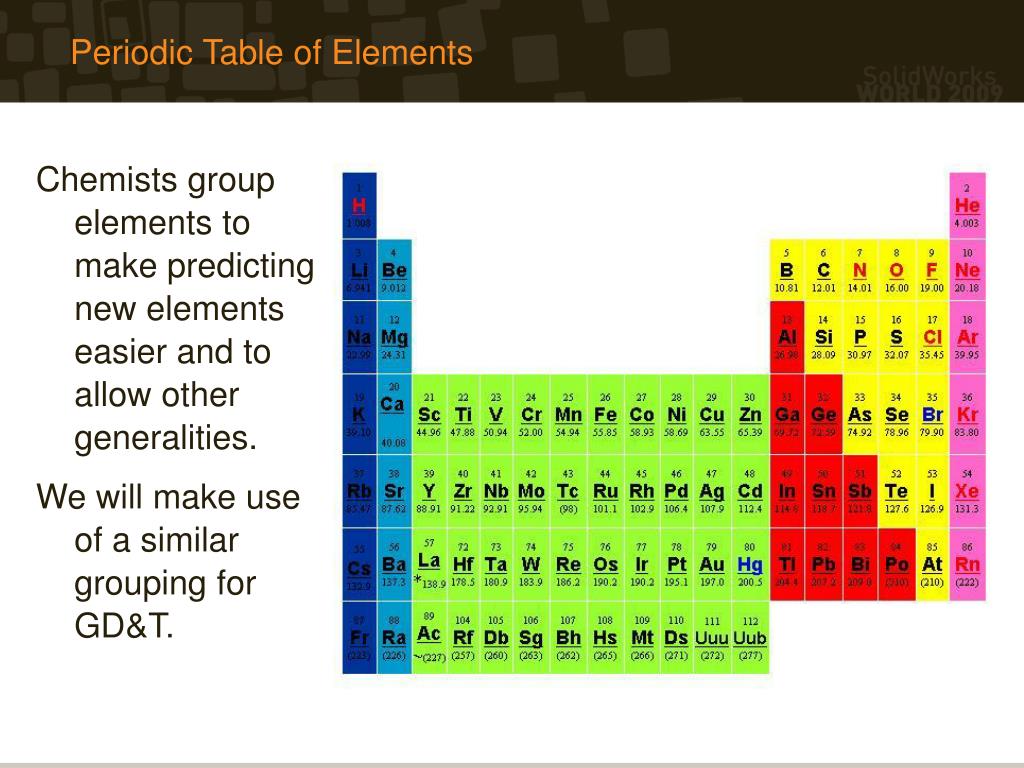
How do you find elements in the periodic table?
How do you find elements in the periodic table? To find the number of electrons an element has, locate it on the periodic table of elements, find the atomic number, and note the number of protons; because atoms are naturally electrically neutral, the protons and electrons are usually equal. Look at the oxidation number for further information.
How can elements be group in periodic table?
What are the three ways of grouping elements in the periodic table?
- Periodic Organization. In the periodic table, an element is defined by its vertical group and horizontal period.
- Scientific Rationale.
- Alkali and Alkaline Earth Metals.
- Transition Metals.
- Metalloids and Nonmetals.
- Noble Gases.
How did Mendeleev arrange elements in periodic table?
Mendeleev arranged the periodic table in order of increasing atomic weight of the elements. He determined that there was a pattern when he arranged these elements into horizontal rows. Mendeleev placed the elements into horizontal rows in his periodic table of elements.

How are the elements in the periodic table arranged?
The Periodic table elements are arranged in the increasing order of their atomic number. The arrangement of elements in the Periodic table starts from the very first top left corner. The first element with atomic number 1 (i.e hydrogen) is placed in the first cell, then gradually the elements with atomic number 2, 3, 4 upto 118, ...
Why is the periodic table arranged by atomic number?
Periodic table is arranged by Atomic number because of electrons present in the outermost orbit (which are responsible for the chemical properties of the elements.)
What is the relationship between the number of protons and the number of electrons?
What is a relationship between number of protons and number of electrons? Simple answer: In a neutral atom, the number of protons and number of electrons are equal. For example, this is a neutral helium atom. Here you can see that the helium atom has 2 protons and the number of electrons are also 2.
What does atomic number mean?
Atomic number = Number of electrons. The electrons present in the outermost orbit represent the chemical properties of the elements. Hence to classify the elements on the basis of similarities in their chemical properties, they are arranged in the Periodic table on the basis of atomic number. In other words, Atomic number indicates the number ...
What are electrons responsible for?
These electrons are responsible for the chemical properties of an element. Hence for grouping the elements according to the similar chemical properties, the elements of the Periodic table are arranged in the increasing order of their atomic number. Later on, we saw that there are groups as well as periods on the Periodic table.
Why are elements 58 to 71 and 90 to 103 in separate rows?
These elements are placed in separate rows at the bottom of the Periodic table because they differ in the chemical properties, plus by placing them at the bottom, ...
How many elements were known in 1869?
During the year 1869, only 63 elements were known. Mendeleev arranged these known elements in the increasing order of their atomic masses. And the Mendeleev’s Periodic table was something like this (as shown below.) But during those years, there was no knowledge about the atomic structure.
How are the columns in the periodic table organized?
The rows and columns are organized by precise characteristics. The elements that are in the same column or in the same rows have common characteristics. For example, magnesium (Mg) and sodium ...
Which two elements are on the top of the periodic table?
The Two Elements At the Top. You are probably wondering about the elements that are alone on the top: hydrogen (H) and helium (He). These two are special elements for different reasons. Hydrogen (H) does not have a single neutron in its neutral form, only one proton and one electron.
Why are valence electrons important?
— Marty Rubin. All the elements in each group have the same number of electrons in their outer orbitals, also known as valence electrons. These electrons are important because they are involved in the chemical bonds with other elements.
How many electrons does helium have?
Helium (He) is unique among all the elements. It only has two electrons in its outer orbital, also known as the valence shell. All the other noble gases (group 18) have eight electrons in their outer orbital or valence shell.
How many orbitals does an element have?
If you look at all the elements on the top row or, in other words, the elements in the first period, you will see that all of them have one atomic orbital for their electrons. Then, the elements on the second row, or second period, are characterized by having two atomic orbitals in their electrons.
How to read valence electrons?
You have to read groups from left to right. All the elements in the first column, or group one, have one valence electrons (one electron in their outer shell). All the elements in the second column, or group two, have two valence electrons. But all the elements in the third group (group three), have thirteen valance electrons. From then on, you have to add an electron for every group until reaching 18. Simply, counting the columns will allow you to know how many electrons each element has on its outer shell. There are a few exceptions to this, though, because some elements are transition elements that add electrons.
Why do all elements have one thing in common?
Because they all have one thing in common: their respective valence shells are full. This is how the periodic table is organized. Understanding that the position of each and every one of the elements is useful in understanding their properties.
What are the elements in the periodic table?
Quick look on Types of Elements on Periodic table 1 Metals#N#Alkali metals#N#Alkaline earth metals#N#Transition metals#N#Inner transition metals 2 Nonmetals 3 Metalloids 4 Families on Periodic table#N#Boron family#N#Carbon family#N#Nitrogen family#N#Chalcogens#N#Halogens#N#Noble gases
How many elements are in the modern periodic table?
In this way, all the 118 known elements are arranged in the Modern Periodic table based on their atomic number.
Why are there two rows at the bottom of the periodic table?
The two rows are placed at the bottom of the Periodic table in order not to distort the shape of Periodic table and also it makes the Periodic table fit perfectly on the A4 size paper.
What are the rows of a periodic table called?
The rows of the Periodic table are known as Periods.
What is the period table?
And it was named as Modern Periodic table. This Periodic table consists of vertical columns called groups and horizontal rows called periods. There are various types of elements on the Periodic table like metals, nonmetals, metalloids, etc. So this is how the Periodic table is arranged.
Where is the atomic number 57?
The element with atomic number 57 is placed in the main part of the Periodic table as shown in below image. Further the elements having atomic number 58, 59, 60, etc are placed in separate rows at the bottom of the Periodic table. This repeats upto element with atomic number 71.
How many groups are there in the periodic table?
There are total 18 groups in modern Periodic table.
How does the periodic table work?
Here's how it works: Elements are listed in numerical order by atomic number. The atomic number is the number of protons in an atom of that element. So element number 1 (hydrogen) is the first element.
How many periods are there in the periodic table?
There are seven periods on the periodic table. Elements in the same period all have the same electron ground state energy level. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties.
What are the trends in the periodic table?
As you progress in chemistry, there are other trends in the periodic table you'll need to know: 1 Atomic radius and ionic radius increase as you move down a group, but decrease as you move across a period. 2 Electron affinity decreases as you move down a group, but increases as you move across a period until you get to the last column. The elements in this group, the noble gases, have practically no electron affinity. 3 The related property, electronegativity, decreases going down a group and increases across a period. Noble gases have practically zero electronegativity and electron affinity because they have complete outer electron shells. 4 Ionization energy decreases as you move down a group, but increases moving across a period. 5 Elements with the highest metallic character are located on the lower left side of the periodic table. Elements with the least metallic character (most nonmetallic) are on the upper right side of the table.
What are the two main types of elements?
The two main types of elements are metals and nonmetals . There are also elements with properties intermediate between metals and nonmetals. These elements are called metalloids or semimetals. Examples of groups of elements that are metals include alkali metals, alkaline earths, basic metals, and transition metals.
Why is the periodic table important?
The periodic table is one of the most valuable tools for chemists and other scientists because it orders the chemical elements in a useful way. Once you understand how the modern periodic table is organized, you'll be able to do much more than just look up element facts like their atomic numbers and symbols.
What is the element symbol?
The element symbol is a shorthand notation that is either one capital letter or a capital letter and a lowercase letter. The exception is the elements at the very end of the periodic table, which have placeholder names (until they are officially discovered and named) and three-letter symbols.
What is the atomic number of an element?
Every atom of hydrogen has 1 proton. Until a new element is discovered, the last element on the table is element number 118 . Every atom of element 118 has 118 protons.
What is the order of the elements in the periodic table?
the elements are listed in order of increasing atomic number . The atomic number is the number of protons in the nucleus of an atom.In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column.
Why are arranged symlinks arranged?
They are arranged to highlight similarites between differnt elements.
How are elements arranged in the periodic table?
In the periodic table the elements are arranged in columns and rows according to increasing atomic number atomic number, often represented by the symbol Z, the number of protons in the nucleus of an atom, as well as the number of electrons in the neutral atom. Atoms with the same atomic number make up a chemical element.
What is the periodic table?
periodic table, chart of the elements arranged according to the periodic law periodic law, statement of a periodic recurrence of chemical and physical properties of the elements when the elements are arranged in order of increasing atomic number.
How many columns are there in the periodic table?
There are 18 vertical columns, or groups, in the standard periodic table. At present, there are three versions of the periodic table, each with its own unique column headings, in wide use. The three formats are the old International Union of Pure and Applied Chemistry (IUPAC) table, the Chemical Abstract Service (CAS) table, and the new IUPAC table. The old IUPAC system labeled columns with Roman numerals followed by either the letter A or B. Columns 1 through 7 were numbered IA through VIIA, columns 8 through 10 were labeled VIIIA, columns 11 through 17 were numbered IB through VIIB and column 18 was numbered VIII. The CAS system also used Roman numerals followed by an A or B. This method, however, labeled columns 1 and 2 as IA and IIA, columns 3 through 7 as IIIB through VIB, column 8 through 10 as VIII, columns 11 and 12 as IB and IIB and columns 13 through 18 as IIIA through VIIIA. However, in the old IUPAC system the letters A and B were designated to the left and right part of the table, while in the CAS system the letters A and B were designated to the main group elements and transition elements respectively. (The preparer of the table arbitrarily could use either an upper-or lower-case letter A or B, adding to the confusion.) Further, the old IUPAC system was more frequently used in Europe while the CAS system was most common in America. In the new IUPAC system, columns are numbered with Arabic numerals from 1 to 18. These group numbers correspond to the number of s, p, and d orbital electrons added since the last noble gas element (in column 18). This is in keeping with current interpretations of the periodic law which holds that the elements in a group have similar configurations of the outermost electron shells of their atoms. Since most chemical properties result from outer electron interactions, this tends to explain why elements in the same group exhibit similar physical and chemical properties. Unfortunately, the system fails for the elements in the first 3 periods (or rows; see below). For example, aluminum, in the column numbered 13, has only 3 s, p, and d orbital electrons. Nevertheless, the American Chemical Society has adopted the new IUPAC system.
What are atoms with the same atomic number?
Atoms with the same atomic number make up a chemical element. Atomic numbers were first assigned to the elements c. ..... Click the link for more information. (see the table entitled Periodic Table Periodic Table of the Elements.
What is the group of elements 89 to 103 called?
Click the link for more information. , or rare earths, and elements 89 to 103 are called the actinide series actinide series , a series of radioactive metallic elements in Group 3 of the periodic table. Members of the series are often called actinides, although actinium (at. no. 89) is not always considered a member of the series.
Which elements in the 18th periodic table have no valence electrons?
The elements grouped in the final column (Group 18) have no valence electrons and are called the inert gases inert gas. or noble gas, any of the elements in Group 18 of the periodic table. In order of increasing atomic number they are: helium, neon, argon, krypton, xenon, and radon.
What is a table of elements?
A table of the elements, written in sequence in the order of atomic number or atomic weight and arranged in horizontal rows (periods) and vertical columns (groups) to illustrate the occurrence of similarities in the properties of the elements as a periodic function of the sequence.
