What are the steps of the periodic table in order?
- H - hydrogen
- He - helium
- Li - lithium
- Be - beryllium
- B - boron
- C - carbon
- N - nitrogen
- O - oxygen
- F - fluorine
How are the elements in the periodic table arranged?
Key points
- The modern periodic table arranges into a unique arrangement that can be used for reference.
- Metals are found on the left of the periodic table and non-metals on the right.
- The periodic table is arranged in rows called periods and columns called groups, which can be used to locate any element.
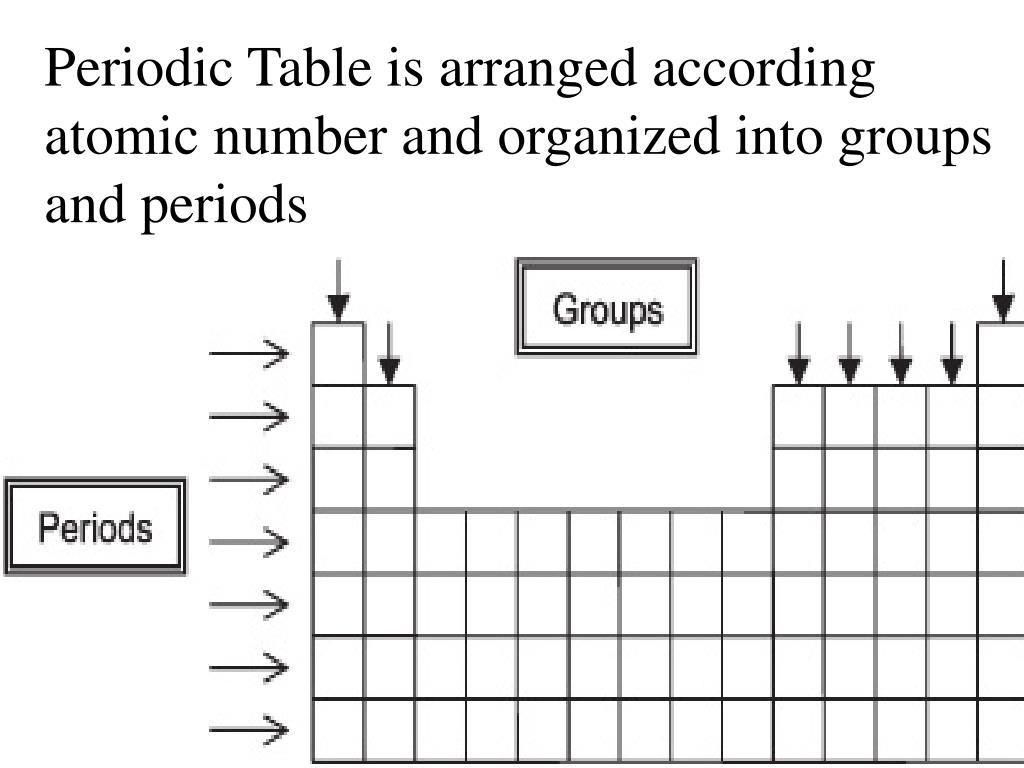
How are periods and groups arranged on the periodic table?
Summary
- The periodic table is arranged in order of atomic number
- A period is a horizontal row of the periodic table.
- A group is a vertical row of the periodic table.
How many groups and periods make up the periodic table?
There are 18 groups, and there are 7 periods plus the lanthanides and actinides. How many groups are in the periodic table? Groups are numbered from 1 to 18.

What are the 3 ways the periodic table is organized?
Here's how it works:Elements are listed in numerical order by atomic number. ... Each horizontal row on the periodic table is called a period. ... Each vertical column on the periodic table is called a group. ... There are two rows of elements found below the main body of the periodic table.More items...•
How is the periodic table arranged?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
How is the periodic table organized and how do we read it?
The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells.
How is the periodic table organized in columns?
A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 3.2. 2). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name.
Why is the periodic table arranged by atomic number?
The number of electrons in an element is fixed. No two elements can have the same atomic number. Hence, elements can be easily classified in the increasing order of their atomic numbers.
How do you explain the periodic table to a child?
The periodic table is a system for arranging the chemical elements. The chemical elements are the basic substances that make up all matter. Each chemical element has a particular feature called its atomic number. That number comes from the amount of tiny particles called protons in each atom of the element.
What is the periodic table sorted by?
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).
Who created the periodic table and how it is organized?
In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them.
What determines the order of elements on today's periodic table?
The elements in the modern periodic table are arranged in order of their atomic numbers, which is the number of protons in the nuclei of the atoms of an element. Each element has a unique atomic number.
Is the periodic table arranged randomly?
The periodic table is organized like a grid. In this article, we will explain how the grid organizes the elements. Understanding how the elements are organized in the grid may help you to learn the periodic table. The position of each of the elements is not at all random but due to its atomic structure.
What are the 7 families of the periodic table?
VocabularyGroup (family): A vertical column in the periodic table.Alkali metals: Group 1A of the periodic table.Alkaline earth metals: Group 2A of the periodic table.Halogens: Group 7A of the periodic table.Noble gases: Group 8A of the periodic table.Transition elements: Groups 3 to 12 of the periodic table.
How do you read the periodic table?
When you're reading the periodic table, move across the table from top left to bottom right. As you move across the table, the number of protons and the atomic mass of each element increases. Each element has its own atomic number, which represents the number of protons in one atom of the element.
How do you read the periodic table of elements?
On the periodic table, elements are listed in order of increasing atomic number.Elements in the same row are in the same period. ... Elements in the same column are in the same group. ... Here's a close-up look at the carbon square from the Periodic Table.
How do you read an element notation?
In elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.
How do you read protons neutrons and electrons on the periodic table?
The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The atomic mass (number at the bottom) is the amount of protons and neutrons added together.