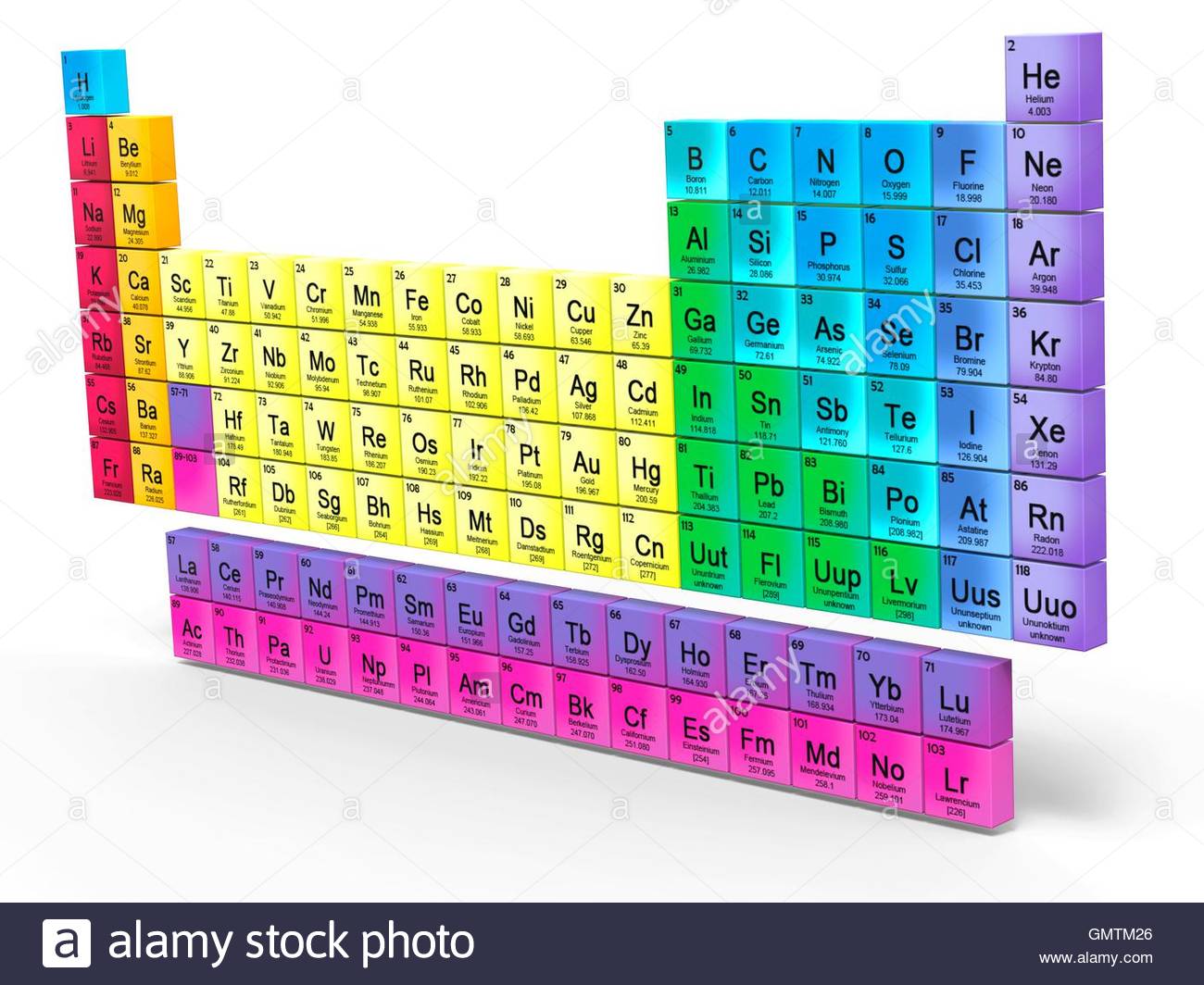
How are the elements arranged in the modern periodic table?
Key points
- The modern periodic table arranges into a unique arrangement that can be used for reference.
- Metals are found on the left of the periodic table and non-metals on the right.
- The periodic table is arranged in rows called periods and columns called groups, which can be used to locate any element.
How groups are there in the modern periodic table?
Mendeleev’s Periodic Table
- Mendeleev stated that the properties of elements are the periodic function of their atomic masses or atomic weights.
- This was the first arrangement of elements in the form of a table.
- Mendeleev arranged elements in horizontal rows and vertical columns, which were called Periods and Groups respectively.
- There were 8 groups and 7 periods in this table.
How many elements are listed in the modern periodic table?
The table below consists of 118 elements of the periodic table, sorted by atomic number, atomic weight, symbols, density, discovered year and the group. There are about ninety elements found on Earth. Each one has a different number of protons, electrons and neutrons.
What is the modern periodic table arranged according to?
The modern periodic table is arranged in ascending order according to atomic number. An element’s atomic number is equal to the number of protons in each atom. Within this order, elements are arranged into distinct groups that share properties.

What are the 3 ways the periodic table is organized?
Here's how it works:Elements are listed in numerical order by atomic number. ... Each horizontal row on the periodic table is called a period. ... Each vertical column on the periodic table is called a group. ... There are two rows of elements found below the main body of the periodic table.More items...•
What is the periodic table organized by?
atomic numberThe periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
How was the periodic table developed and organized?
British chemist John Newlands was the first to arrange the elements into a periodic table with increasing order of atomic masses. He found that every eight elements had similar properties and called this the law of octaves. He arranged the elements in eight groups but left no gaps for undiscovered elements.
Why is the modern periodic table arranged by atomic number?
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).
How the periodic table is Organised in terms of periods and groups?
The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells.
How did Dmitri Mendeleev organize the periodic table?
In his periodic table, Mendeleev arranged elements in rows by increasing atomic mass. Within a row, elements with lower atomic masses were on the left. Mendeleev started a new row every time the chemical properties of the elements repeated. Thus, all the elements in a column had similar properties.
What is Moseley periodic table based on?
atomic numberSolution : Correct Statement : Moseley's periodic table is based on atomic number .
What are the columns of the periodic table called?
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods.
Answer
Elements in a periodic table are arranged according to the increase in atomic number.
New questions in Physics
You have a 12 volt battery and placed across a 6 ohm resistor, what will the current be?
What does the periodic table provide?
The periodic table provides the atomic number, atomic mass, symbol and name. Also provides if the elements are metal,non-metal and the state of matter.
What do periods tell you about Bohr?
Periods tell you the number of energy levels in the Bohr model.
Do metals lose electrons?
Metals have a high density, high melting point, malleable, ductile and good conductors. They lose electrons when combining chemically with nonmetals. The nonmetals are the opposite and are also brittle. They tend to gain electrons when combining chemically with metals. The metalloids are semi-conductors and share the properties of metals and nonmetals.