What do the numbers on the periodic table mean?
What do all the numbers mean on the periodic table? The number above the symbol is the atomic mass (or atomic weight). This is the total number of protons and neutrons in an atom. The number below the symbol is the atomic number and this reflects the number of protons in the nucleus of each element's atom.
What is 6 on the periodic table?
From the periodic table you can see that carbon has an atomic number of 6, which is its proton number. Why is atomic number important? Atomic number is called the number of protons in an atom.
What are the groups on the periodic table?
- Beryllium (Be)
- Magnesium (Mg)
- Calcium (Ca)
- Strontium (Sr)
- Barium (Ba)
- Radium (Ra)
What is N on the periodic table?
The seventh element in the periodic table is nitrogen. The nitrogen atom contains a total of seven electrons and protons. Therefore, the atomic number of nitrogen (N) is 7. Nitrogen is a p-block element and its symbol is ‘N’. This article discusses the properties of the nitrogen element and the importance of the nitrogen atomic number.
See more
What are the 7 periods in periodic table?
Period 7 elementHydrogenHeliumLithiumBerylliumNeonSodiumMagnesiumArgonPotassiumCalciumKryptonRubidiumStrontiumXenon2 more rows
How many periods and groups are in the periodic table?
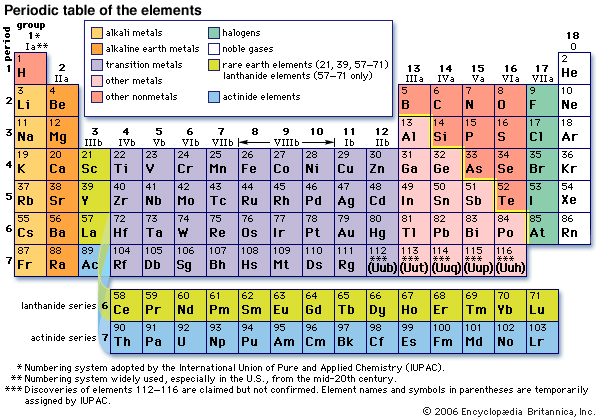
Groups are the columns of the periodic table, and periods are the rows. There are 18 groups, and there are 7 periods plus the lanthanides and actinides.
What is group and period?
The vertical columns in the periodic table are called 'groups' and horizontal rows in the table are called periods.
How many groups are on the periodic table?
18The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons.
Are there 7 or 9 periods on the periodic table?
Periods. There are currently seven complete periods in the periodic table, comprising the 118 known elements.
Are there 8 or 18 groups in the periodic table?
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered.
How do you remember periods and groups?
0:292:53Periods & Groups In The Periodic Table | Properties of Matter - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach new period row represents a new shell elements in the first period have one shell and as we goMoreEach new period row represents a new shell elements in the first period have one shell and as we go down the shells.
What is period number?
The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons.
How do you find the period and group on the periodic table?
To determine the group, we need to understand some rules:If the element is in s block, then the group number is equal to the number of valence electrons. ... If the element is in the p block, then the number of the group can be determined by the formula: (number of valence electrons + 10).for groups.More items...
How many elements are there in the periodic table of 2022?
118Today, with 118 known elements, it is widely regarded as one of the most significant achievements in science.
What are the 8 main groups of the periodic table?
Name of the eight groups in the periodic table:Alkali metals.Alkaline earth metals.Rare earth metals.Crystallogens.Pnictogens.Chalcogens.Halogens.Noble gases.
What are the 5 main groups of the periodic table?
5 Element FamiliesAlkali metals.Alkaline earth metals.Transition metals.Halogens.Noble gases.
Where is the period and group in the periodic table?
The columns of the periodic table are called groups. Members of the same group in the table have the same number of electrons in the outermost shells of their atoms and form bonds of the same type. The horizontal rows are called periods.
How many groups and periods are there in Mendeleev periodic table?
Mendeleev's periodic table consists of sixty three elements arranged in increasing order of their atomic mass. The table consists of eight groups and six periods.
How do you find the period and group of an element?
To determine the group, we need to understand some rules:If the element is in s block, then the group number is equal to the number of valence electrons. ... If the element is in the p block, then the number of the group can be determined by the formula: (number of valence electrons + 10).for groups.More items...
How do you remember periods and groups?
0:292:53Periods & Groups In The Periodic Table | Properties of Matter - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach new period row represents a new shell elements in the first period have one shell and as we goMoreEach new period row represents a new shell elements in the first period have one shell and as we go down the shells.
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
Why is period 1 the shortest period?
Period 1 of the periodic table is given the name shortest period because there are only two elements in period 1.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
What are the elements that chemists classify?
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
What are the different types of nonmetals?
The nonmetals, halogens, and noble gases are all types of nonmetals. The metalloids have properties intermediate between metals and nonmetals. The alkali metals, alkaline earths, lanthanides, actinides, transition metals, and basic metals are all groups of metals.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).