
What do the numbers on the periodic table mean?
What do all the numbers mean on the periodic table? The number above the symbol is the atomic mass (or atomic weight). This is the total number of protons and neutrons in an atom. The number below the symbol is the atomic number and this reflects the number of protons in the nucleus of each element's atom.
What is 6 on the periodic table?
From the periodic table you can see that carbon has an atomic number of 6, which is its proton number. Why is atomic number important? Atomic number is called the number of protons in an atom.
What are the groups on the periodic table?
- Beryllium (Be)
- Magnesium (Mg)
- Calcium (Ca)
- Strontium (Sr)
- Barium (Ba)
- Radium (Ra)
What is N on the periodic table?
The seventh element in the periodic table is nitrogen. The nitrogen atom contains a total of seven electrons and protons. Therefore, the atomic number of nitrogen (N) is 7. Nitrogen is a p-block element and its symbol is ‘N’. This article discusses the properties of the nitrogen element and the importance of the nitrogen atomic number.
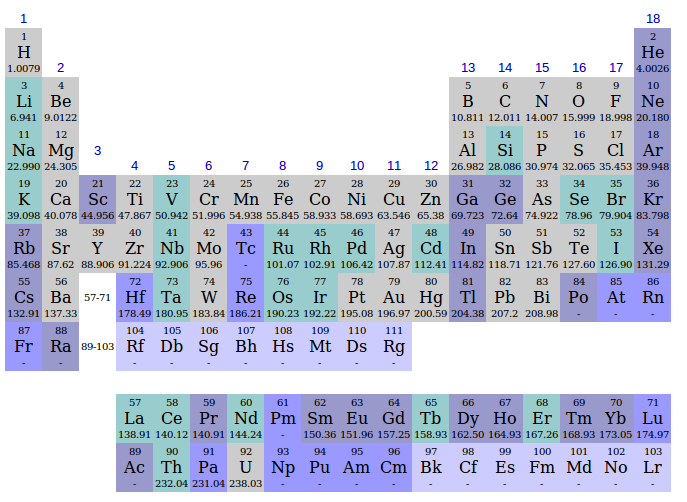
Are there 7 or 9 periods on the periodic table?
Periods. There are currently seven complete periods in the periodic table, comprising the 118 known elements.
What are the 7 periods in periodic table?
Period 7 elementHydrogenHeliumLithiumBerylliumNeonSodiumMagnesiumArgonPotassiumCalciumKryptonRubidiumStrontiumXenon2 more rows
How many periods and groups are there?
Hence, there are 18 groups and 7 periods in the modern periodic table.
What is period 7 called?
Period 7 of the periodic table is the actinides family. The elements having atomic numbers from 89 to 104 come under actinides. They are radioactive metals because their nucleus is highly unstable. Example of actinides is Uranium and Thorium .
Is 7th period complete?
The elements with atomic numbers 113, 115, 117 and 118 will get permanent names soon, according to the International Union of Pure and Applied Chemistry. With the discoveries now confirmed, "The 7th period of the periodic table of elements is complete," according to the IUPAC.
Why there are 7 periods in Mendeleev periodic table?
Elements are arranged in the periodic table in the increasing order of their relative atomic masses. Mendeleev divided his periodic table in eight groups and seven periods. Groups from I to VII are meant for normal elements and group VIII is for transition elements.
What are periods and groups?
A period is a horizontal row of the periodic table. A group is a vertical row of the periodic table.
Which period is the longest in the periodic table?
The sixth periodThe sixth period is called the longest period because it has maximum 32 elements.
Why is the 7th period incomplete?
7th period is considered incomplete because it consists of elements which have not been studied properly yet. Their properties may change after getting sufficient information about them.
What element is in group 8 period 7?
It consists of iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs). They are all transition metals. Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior.
What is period 6 on the periodic table?
The period 6 transition metals are lanthanum (La), hafnium (Hf), tantalum (Ta), tungsten (W), rhenium (Re), osmium (Os), iridium (Ir), platinum (Pt), gold (Au), and mercury (Hg).
Where are the periods on the periodic table?
A period is a horizontal row of the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. A new period begins when a new principal energy level begins filling with electrons.
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
Why is period 1 the shortest period?
Period 1 of the periodic table is given the name shortest period because there are only two elements in period 1.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
What are the elements that chemists classify?
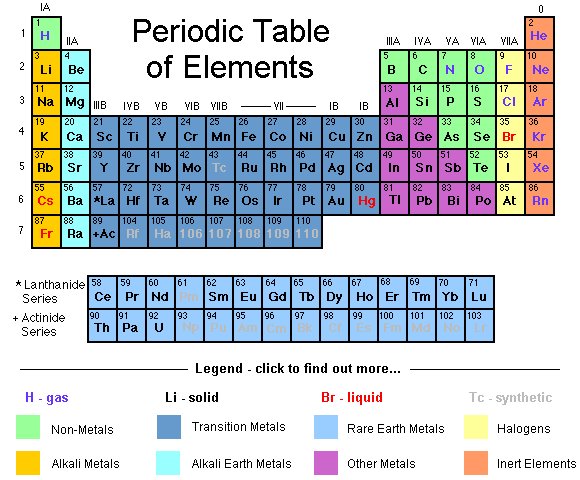
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
What are the different types of nonmetals?
The nonmetals, halogens, and noble gases are all types of nonmetals. The metalloids have properties intermediate between metals and nonmetals. The alkali metals, alkaline earths, lanthanides, actinides, transition metals, and basic metals are all groups of metals.
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
How many categories are there in the periodic table?
The elements of the periodic table shown here are divided into nine categories; six for the metals, and two for nonmetals, and a metalloid category. The nine categories (or sets) correspond to those found in the literature for the applicable part of the periodic table. Different authors may use different categorisation schema depending on the properties of interest.
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
How many electrons are in neon?
The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to ...
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
When is the 150th anniversary of the periodic table?
In celebration of the periodic table's 150th anniversary, the United Nations declared the year 2019 as the International Year of the Periodic Table, celebrating "one of the most significant achievements in science".
What is the atomic number of an element?
The atomic number refers to the number of protons found in the atom of an element . Elements can be categorized into three major groups that include metals, nonmetals, and metalloids. The elements found on the left side of the periodic table are typically metals. While the elements on the right side of the periodic table are non-metals.
How many elements are in pure form?
Thirty-two of the 98 elements are in their pure form. The rest exist as compounds. Eighty of the natural elements are stable, meaning that they cannot be subjected to radioactive decay. Ten of the 98 elements only exist in trace amounts. Typically, all the elements of the periodic table with a higher atomic number than lead are unstable, ...
What does the number of protons in an element mean?
The number of protons in an element gives the atomic number of the element. An element refers to a substance made of atoms of the same kind. All the atoms in a particular element bear the same atomic number. Elements cannot be broken further into smaller substances using chemical reactions. However, they can only be transformed into other elements ...
What are the properties of an element?
The periodic table outlines each element’s electron configuration, the atomic number of the element, and the chemical properties of the element. The atomic number refers to the number of protons found in the atom of an element.
What are the elements that are found naturally?
Non-metals that fall into this category include nitrogen, oxygen, and carbon.
Can an element be broken into smaller substances?
All the atoms in a particular element bear the same atomic number. Elements cannot be broken further into smaller substances using chemical reactions. However, they can only be transformed into other elements by nuclear procedures. The atoms in an element contain the same number of protons, but the number of neutrons varies.
Is a native element a compound?
Native elements, on the other hand, are naturally occurring elements in an uncombined form. However, only a few native elements are found in compound form.
Summary
Overview
The periodic table is a 2-dimensional structured table. The elements are placed in table cells, in reading order of ascending atomic number. The table columns are called groups, the rows are called periods. The breaks at the end of each period occur according to a repetition (or periodicity) of physical and chemical properties of the elements.
Periodic trends
As chemical reactions involve the valence electrons, elements with similar outer electron configurations may be expected to react similarly and form compounds with similar proportions of elements in them. Such elements are placed in the same group, and thus there tend to be clear similarities and trends in chemical behaviour as one proceeds down a group. As analogous configurations return …
Classification of elements
Many terms have been used in the literature to describe sets of elements that behave similarly. The group names alkali metal, alkaline earth metal, pnictogen, chalcogen, halogen, and noble gas are acknowledged by IUPAC; the other groups can be referred to by their number, or by their first element (e.g., group 6 is the chromium group). Some divide the p-block elements from groups 13 to 1…
History
In 1817, German physicist Johann Wolfgang Döbereiner began to formulate one of the earliest attempts to classify the elements. In 1829, he found that he could form some of the elements into groups of three, with the members of each group having related properties. He termed these groups triads. Chlorine, bromine, and iodine formed a triad; as did calcium, strontium, and barium; lithium, sodium, a…
Current questions
Although the modern periodic table is standard today, some variation can be found in period 1 and group 3. Discussion is ongoing about the placements of the relevant elements. The controversy has to do with conflicting understandings of whether chemical or electronic properties should primarily decide periodic table placement, and conflicting views of how the evidence should be used. A similar potential problem has been raised by theoretical investigations of the superheav…
Future extension beyond the seventh period
The most recently named elements – nihonium (113), moscovium (115), tennessine (117), and oganesson (118) – completed the seventh row of the periodic table. Future elements would have to begin an eighth row. These elements may be referred to either by their atomic numbers (e.g. "element 119"), or by the IUPAC systematic element names which directly relate to the atomic n…
Alternative periodic tables
The periodic law may be represented in multiple ways, of which the standard periodic table is only one. Within 100 years of the appearance of Mendeleev's table in 1869, Edward G. Mazurs had collected an estimated 700 different published versions of the periodic table. Many forms retain the rectangular structure, including Janet's left-step periodic table (pictured below), and the m…