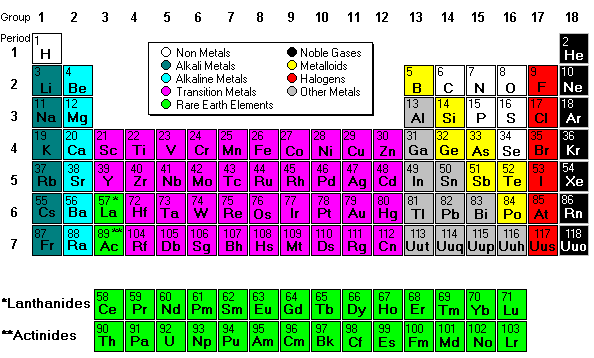
What do the numbers on the periodic table mean?
What do all the numbers mean on the periodic table? The number above the symbol is the atomic mass (or atomic weight). This is the total number of protons and neutrons in an atom. The number below the symbol is the atomic number and this reflects the number of protons in the nucleus of each element's atom.
What is 6 on the periodic table?
From the periodic table you can see that carbon has an atomic number of 6, which is its proton number. Why is atomic number important? Atomic number is called the number of protons in an atom.
What are the groups on the periodic table?
- Beryllium (Be)
- Magnesium (Mg)
- Calcium (Ca)
- Strontium (Sr)
- Barium (Ba)
- Radium (Ra)
What is N on the periodic table?
The seventh element in the periodic table is nitrogen. The nitrogen atom contains a total of seven electrons and protons. Therefore, the atomic number of nitrogen (N) is 7. Nitrogen is a p-block element and its symbol is ‘N’. This article discusses the properties of the nitrogen element and the importance of the nitrogen atomic number.

Are there 7 or 9 periods on the periodic table?
Periods. There are currently seven complete periods in the periodic table, comprising the 118 known elements.
What are the 7 periods in periodic table?
Period 7 elementHydrogenHeliumLithiumBerylliumNeonSodiumMagnesiumArgonPotassiumCalciumKryptonRubidiumStrontiumXenon2 more rows
How many groups and periods are in the periodic table?
Hence, there are 18 groups and 7 periods in the modern periodic table.
What are periods and groups?
The columns of the periodic table are called groups. Members of the same group in the table have the same number of electrons in the outermost shells of their atoms and form bonds of the same type. The horizontal rows are called periods.
What is period 7 called?
Period 7 of the periodic table is the actinides family. The elements having atomic numbers from 89 to 104 come under actinides. They are radioactive metals because their nucleus is highly unstable. Example of actinides is Uranium and Thorium .
Why 7th period is not longest period?
7th period is not the longest as it contains less than 32 elements.
Are there 8 or 18 groups in the periodic table?
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered.
How are periods organized?
The periodic table is arranged by atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row (called a period) and column (called a group).
Which is the oldest element?
PhosphorusThe oldest chemical element is Phosphorus and the newest element is Hassium. Please note that the elements do not show their natural relation towards each other as in the Periodic system.
How do you remember periods and groups?
0:292:53Periods & Groups In The Periodic Table | Properties of Matter - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach new period row represents a new shell elements in the first period have one shell and as we goMoreEach new period row represents a new shell elements in the first period have one shell and as we go down the shells.
What is a period number?
If you are given with the atomic number of an element you can find it's period number and group number. The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons.
What element is in period 2?
The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon.
Is 7th period complete?
The elements with atomic numbers 113, 115, 117 and 118 will get permanent names soon, according to the International Union of Pure and Applied Chemistry. With the discoveries now confirmed, "The 7th period of the periodic table of elements is complete," according to the IUPAC.
What element is in group 8 period 7?
It consists of iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs). They are all transition metals. Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior.
What is period 6 on the periodic table?
The period 6 transition metals are lanthanum (La), hafnium (Hf), tantalum (Ta), tungsten (W), rhenium (Re), osmium (Os), iridium (Ir), platinum (Pt), gold (Au), and mercury (Hg).
How many electron shells are in period 7?
seven electron shellsPeriod 7 elements have electrons in the first seven electron shells. All period 7 elements have one or more electrons in the seventh electron shell (valence electrons). When atomic number increases by one, another electron is added.
How many periods are in the periodic table?
Each horizontal row of elements in the Periodic Table is known as a period.
Which compounds exhibit amphoteric properties?
Oxides that dissolve in both acid and alkali exhibit amphoteric properties.
How to add sodium oxide powder to water?
A small amount of sodium oxide powder is added to 2 cm 3 of distilled water in a test tube. The mixture is stirred well with a glass rod until no further change occurs.
What happens to the atomic radius when going across period 3?
As a result, the atomic radius (atomic size) decreases when going across Period 3. Electronegativity.
How are metallic properties measured?
The metallic properties of an element is measured by its electropositivity.
What period can you use the trend of changes in the properties of the elements?
The trend of changes in the properties of the elements across a period can be used to predict the properties of an element in Period 2 and Period 3.
Why are metals on the left a solid?
Metals on the left are solids because their melting and boiling points are high.
What are the different types of nonmetals?
The nonmetals, halogens, and noble gases are all types of nonmetals. The metalloids have properties intermediate between metals and nonmetals. The alkali metals, alkaline earths, lanthanides, actinides, transition metals, and basic metals are all groups of metals.
What are the elements that chemists classify?
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the CIAAW?
Since 1899 the IUPAC Commission on Isotopic Abundances and Atomic Weights ( CIAAW) has been evaluating atomic weights and abundances. For example, Carbon had an atomic weight of 12.00 in 1902 but today it is [12.0096, 12.0116]! Times sure have changed as the source of the sample will determine the value.
What is PubChem working with?
PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable.
Who is responsible for deciding what needs to be changed?
While much of what is in the periodic table is stable and unlikely to change, the IUPAC organization is responsible for deciding what needs to be changed. They have created criteria for what constitutes the discovery of a new element.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Did Mendeleev's predictions get dismissed?
There were plenty of skeptics and it took years to gain international acceptance, but once newly-discovered elements matched the ones that Mendeleev predicted, his patterns could not be dismissed. In addition, some of the properties that he "fudged" were later recalculated and found to be much closer to his predictions.
Why are valence electrons important?
The reason for this is that the valence electrons, which are the electrons in the outermost shell, are the ones taking part in chemical reactions. These electrons are either donating, accepting, or sharing. Moreover, the more filled the valence shell is, the more stable the element.
What group are alkali metals in?
The Alkali Metals (Group 1) The alkali metals consist of all of the elements in group one with the exception of hydrogen. These elements are extremely reactive and for this reason, are usually found in compounds. In addition, they are water-sensitive (they react violently with water), so they must be stored in oil.
What are noble gases?
The noble gases, also called aerogens, are inert gases. Some examples include argon, krypton, and neon. They can be found in group eighteen on the periodic table. Likewise, this means they have a complete valence shell. For this reason, they are stable and relatively unreactive.
What is an element in the periodic table?
Vocabulary. Elements: A pure substance composed of a single atom. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. Families: Elements that have the same number ...
What is the name of the group of elements that are found in the three states of matter at standard temperature?
The name halogen means “salt formers” in greek. This is evident in nature as halogens interact with metals to form various salts. On another note, the halogens are a unique group of elements. They are the only periodic family that contains elements in the three states of matter at standard temperature. There are 6 halogens and they are located in group 17. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are highly reactive, highly electronegative, and highly toxic non-metals.
Which metals are the second most reactive?
The alkaline earth metals are the second most reactive family on the periodic table (following behind the alkali metals). Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. They are also good thermal and electrical conductors.
How are elements organized in the periodic table?
The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. From this deduction, he formed the periodic table. It is important to note how the location of elements on this table tells us about their properties. A quick way to understand an element’s chemical and physical properties is to know the periodic trends. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. For a more in-depth explanation of periodic trends, click here.