
There are four ways to find the charge of an element:
- Use the periodic table. The usual charge of an element is common to its group. ...
- Use a chart. Charts come from empirical data on the real behavior of elements, which may differ somewhere from the periodic table predictions. ...
- For a single atom, the charge is the number of protons minus the number of electrons.
- Find the charge by balancing charge in a compound.
What are the charges of elements on the periodic table?
There is also a very clear way of knowing whether an element has a positive or a negative ionic charge. All the metallic elements located on the left part of the Periodic Table have a positive ionic charge, while all the metallic elements located on the right part of the Periodic Table have a negative ionic charge.
What are the cations on the periodic table?
firstly there are no cations in a periodic table! But smallest cation that an element of periodic table form is H+. As Hydrogen is the smallest element in periodic table .Hence it forms smallest cation.
Does the periodic table tell the charges of an atom?
To find the ionic charge of an element you'll need to consult your Periodic Table. On the Periodic Table metals (found on the left of the table) will be positive. Non-metals (found on the right) will be negative. But you need to know the specific ionic charge elments. The image below shows the general trend for ionic charge.
Can identify the four blocks of the periodic table?
There are 4 blocks in the periodic table. The blocks are: s-block. p-block. d-block. f-block. All the elements in a block are very similar but each one is slightly different than the other. All of the s-block elements are metals. Generally they are shiny, silvery, good conductors of heat and electricity, and lose their valence electrons easily.
Which part of the periodic table has a positive ionic charge?
How to find ionic charge?
What Is An Ion?
What Are Cations?
How do atoms form ions?
What is the difference between anions and cations?
What do electrons carry with them?
See 4 more
About this website
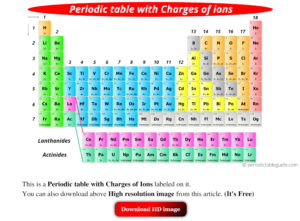
Periodic table with Charges Labeled on it (7 HD Images)
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.; You will get the detailed information about the periodic table which will convert a newbie into pro.; You will also get the HD images of the Periodic table (for FREE).
How to find the charge of an element?
There are four ways to find the charge of an element: Use the periodic table. The usual charge of an element is common to its group. Use a chart. Charts come from empirical data on the real behavior of elements, which may differ somewhere from the periodic table predictions. Here are two charts.
What is the charge of an atom?
These three terms are related, but have slightly different definitions: Charge (Formal Charge): Charge is the electrical charge of an atom when all of its ligands are removed homolytically.
What is the charge of an atom when all of its ligands are removed heterolytically?
Oxidation State (Oxidation Number): Oxidation state is the charge on an atom when all of its ligands are removed heterolytically. In this case, the more electronegative atom gets the electrons. Valence: Valence is the number of electrons used by an atom to form a chemical bond.
Which atoms have an oxidation state of +1?
One electron from each atom participates in chemical bond formation. But, hydrogen has an oxidation state of +1, while chloride has an oxidation state of -1. From the oxidation state, you know the charge (or vice versa). We write the charges of the atoms as H + and Cl –.
Can you predict the charge of an element?
Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group .
Which part of the periodic table has a positive ionic charge?
All the metallic elements located on the left part of the Periodic Table have a positive ionic charge, while all the metallic elements located on the right part of the Periodic Table have a negative ionic charge.
How to find ionic charge?
The best way to find out what the ionic charge for a specific element is is by checking the Periodic table. “Wonder is the heaviest element on the perioid table. Even a tiny fleck of it stops time.”. — Diane Ackerman. There is also a very clear way of knowing whether an element has a positive or a negative ionic charge.
What Is An Ion?
Ion is the name of the subatomic particles that are components of all the atoms. Those particles can be neutrons, which are the neutral subatomic particles located in the very center (nucleus) of the atom together with protons with a positive charge. The isotope of the atom is determined by the number of neutrons and protons therein.
What Are Cations?
Cations are positively charged atoms that are formed from metal atoms. For example, gold, silver copper or sodium. Any electrons that are lost by atoms that are picked up by neutral atoms will turn those neutral atoms into positive atoms.
How do atoms form ions?
The process of ion formation involves atoms giving up electrons in order to form other atoms. This then results in the formation of cations (positively-charged ions) and, also, the atoms then pick up electrons from each other, which results in the formation of anions (negatively-charged ions).
What is the difference between anions and cations?
The main difference between these negatively-charged electrons and cations is that anions do not conduct electricity. The elements in group 13 and group 15 form a cation with a -3 charge each. And elements in group 14 have a charge of -4. Elements in group 16 have a charge of -2, while all the elements of group 17 are halogens with a charge ...
What do electrons carry with them?
Electrons carry with them electrical energy when they move between atoms. If studying the periodic table taught me nothing else, it’s that the credulity of human beings for periodic table panaceas is pretty much boundless. – Sam Kean. If you look at the periodic table, you will find the metals in groups (from one to 16).
