How can you tell valence electrons from the periodic table?
- If the no. of valence electron is 1 to 4 then this is its valency !
- If the no. of valence electron is from 5 to 7 , then the valency is = 8- Valence electrons
- If the no. of valence electrons is 8 , then the valency is 0.
What element has 17 protons and 20 neutrons?
Sulfur has 16 protons, 16 neutrons and 16 electrons: 17: Chlorine has 17 protons, 17 neutrons and 17 electrons: 18: Argon has 18 protons, 22 neutrons and 18 electrons: 19: Potassium has 19 protons, 20 neutrons and 19 electrons: 20: Calcium has 20 protons, 20 neutrons and 20 electrons: 21: Scandium has 21 protons, 24 neutrons and 21 electrons: 22
How many elements can you find on a periodic table?
Unless you currently work in a field that involves science, the days of memorizing the elements of the period table are long behind you. There are currently 118 known elements on the periodic table, and we’ve put 20 of them below to test your smarts.
What side of the periodic table gains electrons?
Because elements on the left side of the periodic table have less than a half-full valence shell, the energy required to gain electrons is significantly higher compared with the energy required to lose electrons. As a result, the elements on the left side of the periodic table generally lose electrons when forming bonds.

What is the periodic table?
The periodic table is a chart that organizes elements by their atomic structure. It is color-coded and assigns each element a unique 1 or 2-letter abbreviation. Other elemental information includes atomic weight and atomic number. You can find a periodic table online or in a chemistry book.
Where is the atomic number located?
The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element. For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
Why is an ion positive?
Although the number of protons in the atom remains the same, the number of electrons is altered in an ion. Because an electron has a negative charge, when you remove electrons, the ion becomes positive.
How to find neutrons?
Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. Remember that the atomic number is the same as the number of protons, which you have already identified.
Why does an ion become positive when you remove electrons?
Because an electron has a negative charge, when you remove electrons, the ion becomes positive. When you add more electrons, the ion becomes negative.
What are the three groups of elements?
Find your element on the periodic table. The table orders elements by atomic number and separates them into three main groups: metals, non-metals, and metalloids (semi-metals). Further elemental groupings include alkali metals, halogens, and noble gases.
Which type of charge is positively charged?
Protons are positively charged, electrons are negatively charged, and neutrons have no (neutral) charge.
How to find valence electrons in a period table?
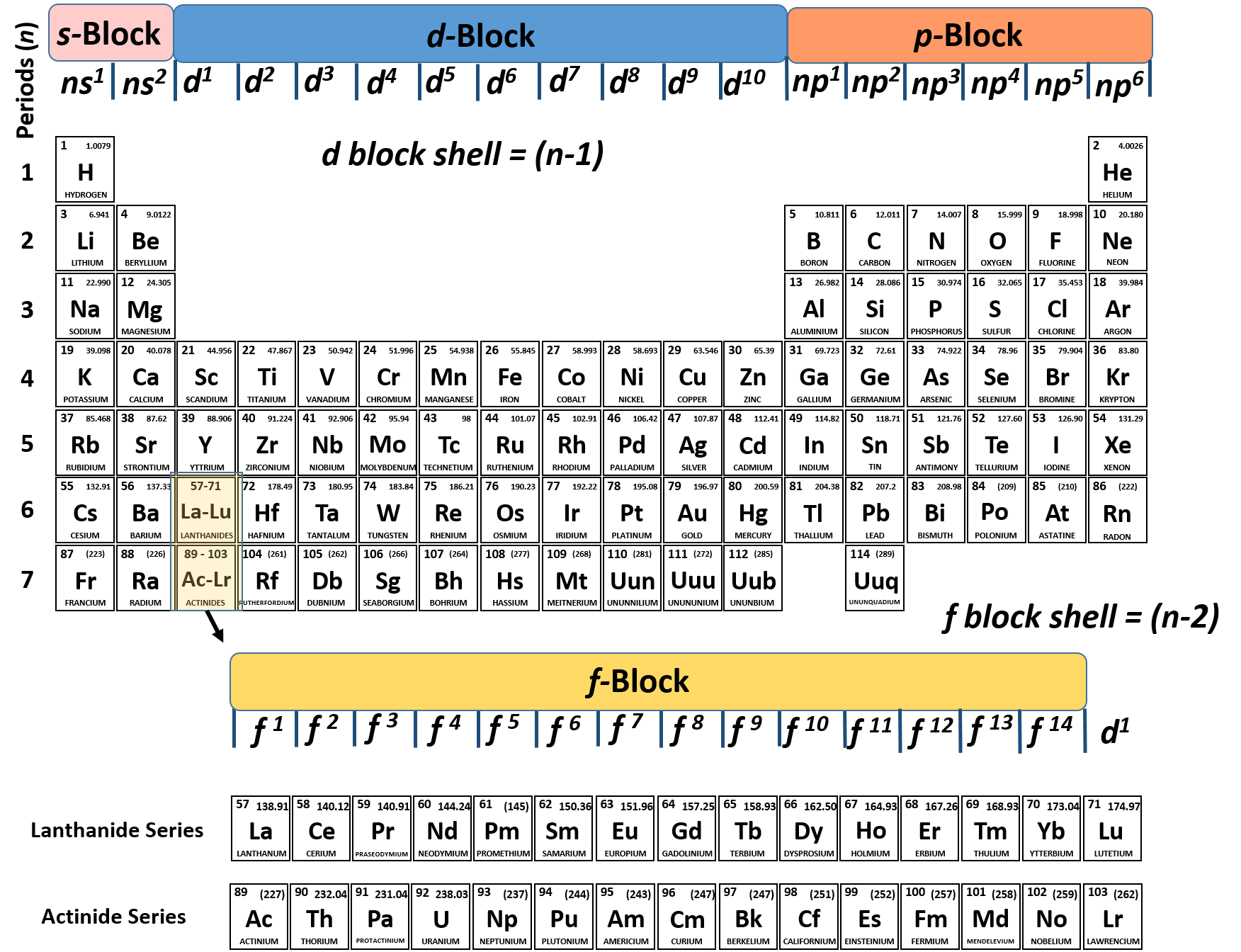
To find valence electrons using a period table, first see if your atom is a transitional metal, which are the elements in the middle rectangle of the table. If the atom is outside this block, locate its group number along the top of the table. The ones digit in the group number is the number of valence electrons.
How to find the number of valence electrons in an atom?
Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones place of the group number is the number of valence electrons in an atom of these elements. In other words:
How many electrons does sodium have?
So, for our example, we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital. That's 11 electrons total — sodium is element number 11, so this makes sense.
How many valence electrons does carbon have?
In our example, since carbon is in group 14, we can say that one atom of carbon has four valence electrons.
How to determine the number of valence electrons?
Determine the number of valence electrons based on the group number. Once again, the group number of the element you are examining can tell you its valence electrons. However, for the transition metals, there isn't a pattern you can follow — group number will usually correspond to a range of possible numbers of valence electrons. These are:
How to label a column on the periodic table?
Label each column on the periodic table of elements from 1 to 18. Generally, on a periodic table, all of the elements in a single vertical column will have the same number of valence electrons. If your periodic table doesn't already have each column numbered, give each a number starting with 1 for the far left end and 18 for the far right end. In scientific terms, these columns are called the element "groups."
How many orbital shells does Selenium have?
For example, we know the element selenium has four orbital shells because it is in the fourth period. Since it is the sixth element from the left in the fourth period (ignoring the transition metals), we know that the outer fourth shell has six electrons, and, thus, that Selenium has six valence electrons.
