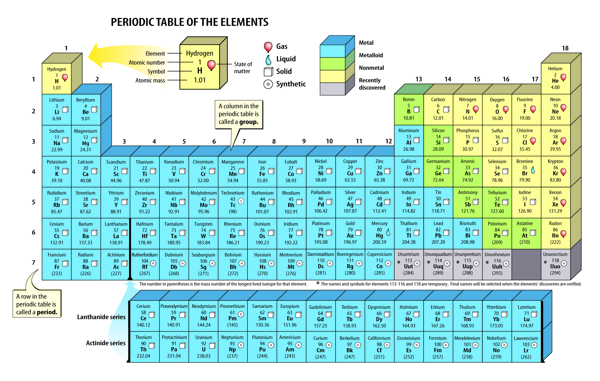
Read the periodic table from top left to bottom right. The elements are ordered by their atomic numbers, which increase as you move across and down the periodic table. The atomic number is how many protons the element’s atom possesses. You’ll also notice that each element’s atomic mass increases as you move across the table.
- Elements in the same row are in the same period. ...
- Elements in the same column are in the same group. ...
- Here's a close-up look at the carbon square from the Periodic Table.
How many elements are listed in periodic table?
How Many Elements Are There?
- There are 118 elements on periodic table
- The number of protons in an element gives the atomic number of the element
- In 2016 four more elements were added into the periodic table
What can we learn about elements from the periodic table?
The periodic table has the following fundamental attributes. There are seven rows or periods. These elements share physical properties, such as malleability and conductivity. Two rows of elements from the atomic number 57 to 71 (lanthanides) and 89 to 103 (actinides) are usually grouped below the main table. There are 18 columns or groups.
How are elements put in order in the periodic table?
- The groups are not divided into sub-groups.
- The elements present in a group have the same number of valence electrons and valency.
- The number of shells increases as we go down the group.
- The elements present in a group have identical chemical properties and their physical properties like density, melting point vary gradually.
How do elements on the periodic table get their names?
Key Takeaways: How Elements Are Named
- Official element names and symbols are determined by the International Union of Pure and Applied Chemistry (IUPAC).
- However, elements often have common names and symbols in various countries.
- Elements don't gain official names and symbols until after their discovery has been verified. ...
- Some element groups have naming conventions. ...

How do you read the periodic table for dummies?
0:073:07The Periodic Table Explained - YouTubeYouTubeStart of suggested clipEnd of suggested clipFirst the letters are symbols. Each box represents an element each element is made up of the sameMoreFirst the letters are symbols. Each box represents an element each element is made up of the same kind of atom with a specific number of protons in its nucleus.
How do you read elements easily?
When you're reading the periodic table, move across the table from top left to bottom right. As you move across the table, the number of protons and the atomic mass of each element increases. Each element has its own atomic number, which represents the number of protons in one atom of the element.
How do you read the periodic table of electrons?
The easiest way to find the number of protons, neutrons, and electrons for an element is to look at the element's atomic number on the periodic table. That number is equal to the number of protons. The number of protons is equal to the number of electrons, unless there's an ion superscript listed after the element.
How do you read the group and period of an element?
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
What is the fastest way to memorize the periodic table?
1. Repetition - How to Memorize the Periodic Table SlowlyMemorize chunks of five elements at a time. You'll have 23 separate groups of five to learn, with a few left over. ... Recite the element names out loud. ... Space out your learning sessions. ... Aim to overlearn.
How can I memorize the periodic table of elements 9?
Break the periodic table into small sections – Divide it into type and colour to find patterns that you may remember. Go back to it when you're free – In the time you have when you're doing not much else pick up the periodic table and give it a look. Do not give up on it. Stay dedicated.
How do you figure out protons neutrons and electrons?
Calculating numbers of subatomic particles To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number: number of protons = atomic number. number of electrons = atomic number. number of neutrons = mass number - atomic number.
How do you read the atomic number?
0:072:23Understanding Atomic Number and Atomic Mass - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe number that appears below the element symbol is called the atomic mass the mass of an atomMoreThe number that appears below the element symbol is called the atomic mass the mass of an atom depends on the number of protons neutrons.
How do you find protons in an element?
Finding the Number of Protons The number of protons in an atom is equal to the atomic number of the element. For example, let's use oxygen. According to the periodic table, oxygen has the atomic number eight. The atomic number is located above the element's symbol.
How do you know which group an element belongs to?
To determine the group, we need to understand some rules:If the element is in s block, then the group number is equal to the number of valence electrons. ... If the element is in the p block, then the number of the group can be determined by the formula: (number of valence electrons + 10).for groups.More items...
What do the numbers mean on a periodic table?
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).
How do u find neutrons?
To find the number of neutrons, subtract the number of protons from the mass number. number of neutrons=40−19=21.
What is the element key?
What is the Element Key? The box containing each element's information is known as the element key. Each key contains an element's name, unique symbol, atomic weight and atomic number. Oxygen, for example, has an atomic number of 8, an atomic weight of 15.996 and a unique symbol, O.
How do you find the protons neutrons and electrons?
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number: number of protons = atomic number. number of electrons = atomic number. number of neutrons = mass number - atomic number.
How do you read isotopes?
0:277:31Isotope Notation - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo here we have 12 protons in the nucleus an atomic number of 12. The number up here is called theMoreSo here we have 12 protons in the nucleus an atomic number of 12. The number up here is called the mass number which is the number of protons. Plus the number of neutrons.
What order are elements listed on the periodic table?
On the periodic table, elements are listed in order of increasing atomic number.
How to keep track of elements?
To keep track of the elements, scientists use the Periodic Table, a chart that shows all the elements. ( Click here for a pdf version of the Periodic Table.) Scientists can quickly find out basic information about an element just by looking at the Periodic Table.
What is the atomic number of an atom?
Atomic Number: the number of protons in the nucleus (which is the same as the number of electrons in the atom).
What does it mean when elements are in the same column?
Elements in the same column are in the same group. This means they react with other elements in similar ways.
What is the periodic table?
Periodic Table An arrangement of the elements by their properties and their atomic number. Element A substance that cannot be divided into simpler substances by chemical means. Symbol A one- or two-letter abbreviation used to represent an element. The first letter is capitalized. Any second letter is always lower case.
Where is the atomic number of an element?
The atomic number of the element is represented by the number in the upper right-hand corner of the entry. This number represents the number of protons in the nucleus of the atom which determines the element. For this example, the atomic number is 12.
What is an element?
Element A substance that cannot be divided into simpler substances by chemical means.
What is the atomic mass of magnesium?
It has an atomic number of 12 and an atomic mass of 24.305 amu.
What is an element in the periodic table?
Vocabulary. Elements: A pure substance composed of a single atom. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. Families: Elements that have the same number ...
How are elements organized in the periodic table?
The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. From this deduction, he formed the periodic table. It is important to note how the location of elements on this table tells us about their properties. A quick way to understand an element’s chemical and physical properties is to know the periodic trends. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. For a more in-depth explanation of periodic trends, click here.
Why are valence electrons important?
The reason for this is that the valence electrons, which are the electrons in the outermost shell, are the ones taking part in chemical reactions. These electrons are either donating, accepting, or sharing. Moreover, the more filled the valence shell is, the more stable the element.
What are noble gases?
The noble gases, also called aerogens, are inert gases. Some examples include argon, krypton, and neon. They can be found in group eighteen on the periodic table. Likewise, this means they have a complete valence shell. For this reason, they are stable and relatively unreactive.
What is the name of the group of elements that are found in the three states of matter at standard temperature?
The name halogen means “salt formers” in greek. This is evident in nature as halogens interact with metals to form various salts. On another note, the halogens are a unique group of elements. They are the only periodic family that contains elements in the three states of matter at standard temperature. There are 6 halogens and they are located in group 17. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are highly reactive, highly electronegative, and highly toxic non-metals.
Which metals are the second most reactive?
The alkaline earth metals are the second most reactive family on the periodic table (following behind the alkali metals). Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. They are also good thermal and electrical conductors.
How many periods are there in the periodic table?
Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases.
Do you ever wonder what makes up the world around you?
Everything you can think of is made up of one or more elements — the chemical building blocks of the universe.
Elements
Just like when you use Google Maps to find the address of a site you want to visit, you can use a square on the periodic table to learn basic information about an element.
Groups
Groups appear as columns numbered from 1-18, from left to right at the top of the table.
Periods
Periods appear as rows numbered from 1-7, from top to bottom on the left side of the table.
Take Action
You don't need to know every element, but getting some practice with the periodic table will help you become more familiar with the elements you need to know in class.
What is periodic table?
1. A copy of the Periodic Table of Elements with a color coordinated legend. 2. You will need a computer with internet access if you do not own a copy. 3.
Why are elements in the periodic table arranged in vertical groups?
These are known as groups. The elements are arranged in these vertical groups because they share similar properties with each other.
What does the atomic number represent?
The number on the very top is called the atomic number and it represents the number of protons in a single atom of the element. For the element helium (shown above) the atomic number is two. The letters in each block represent the atomic symbol. This is essentially an abbreviation of the ...
Which element has the first atomic number?
Hydrogen (H) is the first element with an atomic number of 1. Beginning with hydrogen in the top left of the table, atomic numbers increase from left to right and top to bottom. However, this ordering is not indicative of the size of the elements. Ask Question.
Which element is the smallest in the atomic radius?
Atomic radius is ordered beginning in the top right of the table with helium (He) being the smallest element. Size increases on the table from right to left and top to bottom with the element in the bottom left of the table (francium of Fr) being the largest of all the elements. Ask Question.
Our Periodic Table of Elements Content
Here is a snapshot of the periodic table of elements we have created for you.
How to Read a Periodic Table of Elements
A periodic table of elements places and groups the elements which existence we have identified based on their nature. You may notice some gaps that the periodic table of elements has in the middle. That is because we place the transition metals group there which nature is quite different from other elements.
Elements Grouping and Classification in a Periodic Table of Elements
As you can see in one of our periodic table of elements legend, the table divides the elements into ten groups. They are the alkali metals, alkali earth metals, transition metals, post-transition metals, metalloids, nonmetals, halogens, noble gases, lanthanides, and actinides.
How to Create the Table in Excel
Want to learn how to create a periodic table of elements in excel? It is very simple and you should be able to do it if you have mastered the basic functions in excel!
How to Modify this Table in Its Excel Template
Want to modify the display of our periodic table of elements in its excel file? You should be able to do most things we did to create the table beforehand!
Additional Note
To retrieve the information about an element from our periodic table in excel, you can use the HLOOKUP or INDEX MATCH. You may want to create a separate table first though that contains all the elements information you need. This is so you can retrieve the information you need later much easier.

CORE Concepts
Related Articles
Vocabulary
- Elements: A pure substance composed of a single atom.
- Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element.
- Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element.
- Elements: A pure substance composed of a single atom.
- Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element.
- Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element.
- Families: Elements that have the same number of valence electrons and therefore similar properties.
The Periodic Table and The Periodic Trends
- The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed...
Periods on The Periodic Table
- So what is a period on the periodic table? Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases. Below is a table to help visuals the periodic nu…
Groups of The Periodic Table
- As previously mentioned, the vertical columns on the periodic table are called “groups”. There is eighteen groups on the periodic table in total, and each periodic table group contains elements with the same number of valence electrons. The number of valence electrons present dictates the properties of an element. The reason for this is that the valence electrons, which are the electro…
Families of The Periodic Table
- On the periodic table, there are familieswhich are groups of elements with similar properties. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. Many of these families belong to a single group on the periodic table. However, not all of the families overlap with periodic table groups. F…