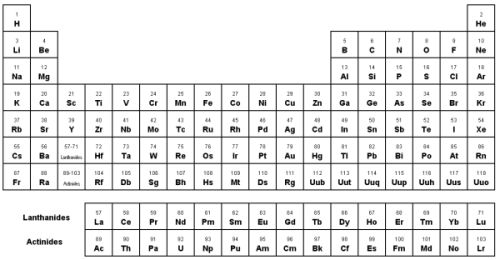
- Elements in the same row are in the same period. ...
- Elements in the same column are in the same group. ...
- Here's a close-up look at the carbon square from the Periodic Table.
What is the easiest way to learn the periodic table?
- Try and picture something for each element. For example, for hydrogen picture a star, or for uranium picture a power plant. ...
- Memorize in chunks or five, then each day add 5 elements. Do that for 23 days more or less and you have memorized 115 elements, basically the periodic table.
- The third method isn’t really a method. ...
What is 101 on the periodic table?
face-centered cubic (fcc) Mendelevium is a synthetic element with the symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranic element in the actinide series, it is the first element by atomic number that currently cannot be produced in macroscopic quantities through neutron bombardment of lighter elements.
What are some interesting facts about the periodic table?
Interesting Facts On Periodic Table of Elements
- Founder of Periodic Table. Dmitri Mendeleyev is the father of the modern periodic table of elements. ...
- Columns of the Periodic Table. The periodic table has 18 vertical columns called groups and seven horizontal columns called Periods.
- Size of the Atom. ...
- Unique Elements. ...
- Properties of Elements. ...
- Facts About Hydrogen. ...
How do you learn the periodic table?
on How To Learn The Periodic Table Of Elements? (Best solution) Break down the table into sections. Spread out the memorization process. Learn the elements in a song. Make nonsense words made from element symbols. Use color to learn element groups. Use a mnemonic device to help remember the order of the elements.

How do you read the periodic table for dummies?
0:073:07The Periodic Table Explained - YouTubeYouTubeStart of suggested clipEnd of suggested clipFirst the letters are symbols. Each box represents an element each element is made up of the sameMoreFirst the letters are symbols. Each box represents an element each element is made up of the same kind of atom with a specific number of protons in its nucleus.
What do the numbers mean on a periodic table?
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).
How do you read the periodic table of electrons?
The easiest way to find the number of protons, neutrons, and electrons for an element is to look at the element's atomic number on the periodic table. That number is equal to the number of protons. The number of protons is equal to the number of electrons, unless there's an ion superscript listed after the element.
What is the periodic table for beginners?
The periodic table of the elements – or just the periodic table – is a table that displays all of the elements. As there are 118 known elements (at this point), it is important that they be organised in a convenient and sensible way.
How do you read groups and periods on a periodic table?
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
How can I memorize the periodic table?
Memorization StrategiesBreak down the table into sections. ... Spread out the memorization process. ... Learn the elements in a song. ... Make nonsense words made from element symbols. ... Use color to learn element groups. ... Use a mnemonic device to help remember the order of the elements.
How do you read the atomic number?
0:072:23Understanding Atomic Number and Atomic Mass - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe number that appears below the element symbol is called the atomic mass the mass of an atomMoreThe number that appears below the element symbol is called the atomic mass the mass of an atom depends on the number of protons neutrons.
How is the periodic table arranged?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
Are protons and electrons equal?
The number of electrons on a neutral atom is equal to the number of protons in the nucleus of the atom. This is known as the atomic number, Z. The removal or addition of electrons to a neutral atom creates ions that have a net negative or positive charge.
How do you use a period table?
The key to knowing how to use a periodic table is understanding its organization:Elements are listed in order of increasing atomic number. ... Elements are grouped according to periodic properties or trends. ... A row of the periodic table is called an element period. ... A column of the periodic table is called an element group.More items...•
How do you teach children the periodic table?
5 Fun Ways to Teach the Periodic TableTry a merka Learning Kit. ... Go Over It Each Day (in Song) ... Play an Educational App. ... Use Fun Fact Flash Cards. ... Build Your Own with Post-It Notes.
What are chemistry basics?
The Fundamentals of Chemistry is an introduction to the Periodic Table, stoichiometry, chemical states, chemical equilibria, acid & base, oxidation & reduction reactions, chemical kinetics, inorganic nomenclature and chemical bonding.
What does 2 mean on an element?
Helium is the element that is atomic number 2 on the periodic table. Each helium atom has 2 protons in its atomic nucleus. The atomic weight of the element is 4.0026.
What do the numbers on the periodic chart mean quizlet?
The number (usually placed) above the element symbol, that tells you how many protons, and how many electrons are in the atom.
What number in the periodic table tells you the number of electrons?
Look up the atomic number in the Periodic Table - making sure that you choose the right number if two numbers are given. The atomic number will always be the smaller one. This tells you the number of protons, and hence the number of electrons.
How many periods are there in the periodic table?
Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases.
What is an element in the periodic table?
Vocabulary. Elements: A pure substance composed of a single atom. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. Families: Elements that have the same number ...
What group are alkali metals in?
The Alkali Metals (Group 1) The alkali metals consist of all of the elements in group one with the exception of hydrogen. These elements are extremely reactive and for this reason, are usually found in compounds. In addition, they are water-sensitive (they react violently with water), so they must be stored in oil.
What are noble gases?
The noble gases, also called aerogens, are inert gases. Some examples include argon, krypton, and neon. They can be found in group eighteen on the periodic table. Likewise, this means they have a complete valence shell. For this reason, they are stable and relatively unreactive.
What is the name of the group of elements that are found in the three states of matter at standard temperature?
The name halogen means “salt formers” in greek. This is evident in nature as halogens interact with metals to form various salts. On another note, the halogens are a unique group of elements. They are the only periodic family that contains elements in the three states of matter at standard temperature. There are 6 halogens and they are located in group 17. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are highly reactive, highly electronegative, and highly toxic non-metals.
Which metals are the second most reactive?
The alkaline earth metals are the second most reactive family on the periodic table (following behind the alkali metals). Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. They are also good thermal and electrical conductors.
How are elements organized in the periodic table?
The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. From this deduction, he formed the periodic table. It is important to note how the location of elements on this table tells us about their properties. A quick way to understand an element’s chemical and physical properties is to know the periodic trends. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. For a more in-depth explanation of periodic trends, click here.
What order are elements listed on the periodic table?
On the periodic table, elements are listed in order of increasing atomic number.
How to keep track of elements?
To keep track of the elements, scientists use the Periodic Table, a chart that shows all the elements. ( Click here for a pdf version of the Periodic Table.) Scientists can quickly find out basic information about an element just by looking at the Periodic Table.
What is the atomic number of an atom?
Atomic Number: the number of protons in the nucleus (which is the same as the number of electrons in the atom).
What does it mean when elements are in the same column?
Elements in the same column are in the same group. This means they react with other elements in similar ways.
What is the periodic table?
Periodic Table An arrangement of the elements by their properties and their atomic number. Element A substance that cannot be divided into simpler substances by chemical means. Symbol A one- or two-letter abbreviation used to represent an element. The first letter is capitalized. Any second letter is always lower case.
Where is the atomic number of an element?
The atomic number of the element is represented by the number in the upper right-hand corner of the entry. This number represents the number of protons in the nucleus of the atom which determines the element. For this example, the atomic number is 12.
What is an element?
Element A substance that cannot be divided into simpler substances by chemical means.
What is the atomic mass of magnesium?
It has an atomic number of 12 and an atomic mass of 24.305 amu.
How To Read The Periodic Table?
There are various ways in which the elements in the periodic table are placed and characterized. An element can be considered a part of a row and column with further sub-classifications across rows, columns, or the table. Therefore, the interpretations are vast and could vary based on one’s purpose. Below we explain the basic forms of classification of elements in the periodic table.
How are elements placed in the periodic table?
An element can be considered a part of a row and column with further sub-classifications across rows, columns, or the table. Therefore, the interpretations are vast and could vary based on one’s purpose. Below we explain the basic forms of classification of elements in the periodic table.
What are metalloids called?
Metalloids: These are also called semi-metals in chemistry. Metalloid elements have characteristics that lie in between metals and non-metals. For instance, metalloids could be shiny like metals but could be poor conductors of heat like non-metals. Some metalloids could be malleable like metals, while others could have poor malleability like non-metals.
What is the most common basis for the classification of elements in the periodic table?
In the modern periodic table, elements are listed based on their atomic number, which is the most common basis for their categorization and place on the table.
What are the most recently added elements to the periodic table?
The most recently added elements to the periodic table were Nihonium, Moscovium, Tennessine, and Oganesson. These elements were added in 2016 after a five-month review by the International Union of Pure and Applied Chemistry (IUPAC).
How many columns are there in the periodic table?
The elements in the periodic table are arranged in rows and columns. There are 18 columns known as groups and seven rows called periods. The periodic table gets its name from the seven periods (2).
Why was the periodic table created?
The periodic table was designed to accommodate elements that were discovered later. New elements can be categorized and positioned in the existing periodic table. In fact, many elements present in the modern periodic table were not part of the original periodic table in 1869.

CORE Concepts
Related Articles
Vocabulary
- Elements: A pure substance composed of a single atom.
- Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element.
- Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element.
- Elements: A pure substance composed of a single atom.
- Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element.
- Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element.
- Families: Elements that have the same number of valence electrons and therefore similar properties.
The Periodic Table and The Periodic Trends
- The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed...
Periods on The Periodic Table
- So what is a period on the periodic table? Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases. Below is a table to help visuals the periodic nu…
Groups of The Periodic Table
- As previously mentioned, the vertical columns on the periodic table are called “groups”. There is eighteen groups on the periodic table in total, and each periodic table group contains elements with the same number of valence electrons. The number of valence electrons present dictates the properties of an element. The reason for this is that the valence electrons, which are the electro…
Families of The Periodic Table
- On the periodic table, there are familieswhich are groups of elements with similar properties. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. Many of these families belong to a single group on the periodic table. However, not all of the families overlap with periodic table groups. F…
How to Read The Periodic table?
- Vocabulary
1. Elements:Substances consisting of only one atom. 2. Groups:The vertical column of the periodic table signifies the number of valence electrons in an element. 3. Periods:The horizontal rows in a periodic table indicate the number of electron shells in an element. 4. Families:Elemen…
The Periodic Table and The Periodic Trends
- A periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of similar elements). The number of electrons in the valence electrons of elements belong to the same group. In the meantime, elements in the same period have the same number of occupied electron shells. Russian chemist Dmitri Mendeleev noticed there was an inn…
Periods on The Periodic Table
- What is a period on the periodic table? Periodsare the horizontal rows of a periodic table. In total, there are seven periods, each with the same number of atomic orbitals. There are only two orbitals in the maximum period, which contains hydrogen and helium. The number of orbitals increases as you move down the rows.
Groups of The Periodic Table
- As previously mentioned, the vertical columns on the periodic table are called “groups.” There are eighteen groups on the periodic table in total, and each periodic table groupcontains elements with the same number of valence electrons. Element properties are determined by the number of valence electrons present. This is because valence electrons, which are electrons in the outerm…
Families of The Periodic Table
- On the periodic table, some families are groups of elements with similar properties. Alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases comprise these families. On the periodic table, most of these families are in one group. There are, however, some families that overlap with periodic table groups. As an example…