- Elements in the same row are in the same period. ...
- Elements in the same column are in the same group. ...
- Here's a close-up look at the carbon square from the Periodic Table.
How many elements are listed in periodic table?
How Many Elements Are There?
- There are 118 elements on periodic table
- The number of protons in an element gives the atomic number of the element
- In 2016 four more elements were added into the periodic table
What are some of the elements from the periodic table?
There are eleven elements represented in the periodic table by letters not in line with their names:
- Sodium (Na – Natrium)
- Potassium (K – Kalium)
- Iron (Fe – Ferrum)
- Copper (Cu – Cuprum)
- Silver (Ag – Argentum)
- Tin (Sn – Stannum)
- Antimony (Sb – Stibium)
- Tungsten (W – Wolfram)
- Gold (Au – Aurum)
- Mercury (Hg – Hydrargyrum)
What can we learn about elements from the periodic table?
The periodic table has the following fundamental attributes. There are seven rows or periods. These elements share physical properties, such as malleability and conductivity. Two rows of elements from the atomic number 57 to 71 (lanthanides) and 89 to 103 (actinides) are usually grouped below the main table. There are 18 columns or groups.
How are elements put in order in the periodic table?
- The groups are not divided into sub-groups.
- The elements present in a group have the same number of valence electrons and valency.
- The number of shells increases as we go down the group.
- The elements present in a group have identical chemical properties and their physical properties like density, melting point vary gradually.

What order are elements listed on the periodic table?
On the periodic table, elements are listed in order of increasing atomic number.
How to keep track of elements?
To keep track of the elements, scientists use the Periodic Table, a chart that shows all the elements. ( Click here for a pdf version of the Periodic Table.) Scientists can quickly find out basic information about an element just by looking at the Periodic Table.
What is the atomic number of an atom?
Atomic Number: the number of protons in the nucleus (which is the same as the number of electrons in the atom).
What does it mean when elements are in the same column?
Elements in the same column are in the same group. This means they react with other elements in similar ways.
What is an element in the periodic table?
Vocabulary. Elements: A pure substance composed of a single atom. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. Families: Elements that have the same number ...
How are elements organized in the periodic table?
The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. From this deduction, he formed the periodic table. It is important to note how the location of elements on this table tells us about their properties. A quick way to understand an element’s chemical and physical properties is to know the periodic trends. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. For a more in-depth explanation of periodic trends, click here.
Why are valence electrons important?
The reason for this is that the valence electrons, which are the electrons in the outermost shell, are the ones taking part in chemical reactions. These electrons are either donating, accepting, or sharing. Moreover, the more filled the valence shell is, the more stable the element.
What are noble gases?
The noble gases, also called aerogens, are inert gases. Some examples include argon, krypton, and neon. They can be found in group eighteen on the periodic table. Likewise, this means they have a complete valence shell. For this reason, they are stable and relatively unreactive.
What is the name of the group of elements that are found in the three states of matter at standard temperature?
The name halogen means “salt formers” in greek. This is evident in nature as halogens interact with metals to form various salts. On another note, the halogens are a unique group of elements. They are the only periodic family that contains elements in the three states of matter at standard temperature. There are 6 halogens and they are located in group 17. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are highly reactive, highly electronegative, and highly toxic non-metals.
Which metals are the second most reactive?
The alkaline earth metals are the second most reactive family on the periodic table (following behind the alkali metals). Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. They are also good thermal and electrical conductors.
How many periods are there in the periodic table?
Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases.
What is periodic table?
1. A copy of the Periodic Table of Elements with a color coordinated legend. 2. You will need a computer with internet access if you do not own a copy. 3.
Why are elements in the periodic table arranged in vertical groups?
These are known as groups. The elements are arranged in these vertical groups because they share similar properties with each other.
What does the atomic number represent?
The number on the very top is called the atomic number and it represents the number of protons in a single atom of the element. For the element helium (shown above) the atomic number is two. The letters in each block represent the atomic symbol. This is essentially an abbreviation of the ...
Which element has the first atomic number?
Hydrogen (H) is the first element with an atomic number of 1. Beginning with hydrogen in the top left of the table, atomic numbers increase from left to right and top to bottom. However, this ordering is not indicative of the size of the elements. Ask Question.
Which element is the smallest in the atomic radius?
Atomic radius is ordered beginning in the top right of the table with helium (He) being the smallest element. Size increases on the table from right to left and top to bottom with the element in the bottom left of the table (francium of Fr) being the largest of all the elements. Ask Question.
What is periodic table?
The periodic table is a graphical collection of element data. The table lists the chemical elements in order of increasing atomic number, which is the number of protons in an atom of an element. The rows (periods) and columns (groups) organize elements according to similar properties. For example, all of the elements in ...
Why is the periodic table given a range?
When a range is given, it's because the abundance of isotopes varies from one sampling location to another.
What is the atomic number of an element?
The atomic number is how many protons an atom of that element contains. The number of protons is the deciding factor when distinguishing one element from another. Variation in the number of electrons or neutrons does not change the type of element.
What are the vertical columns in the periodic table called?
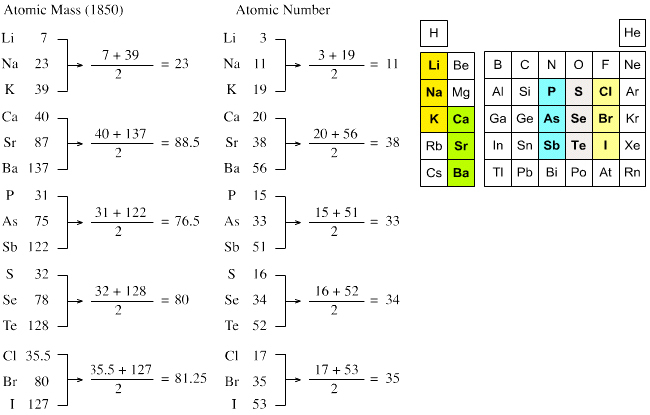
Mendeleev's original periodic table organized elements in order of increasing atomic mass or weight. The vertical columns are called groups. Each element in a group has the same number of valence electrons and typically behave in a similar manner when bonding with other elements. The horizontal rows are called periods.
What happens when the number of electrons changes?
Changing number of electrons produces ions while changing the number of neutrons produces isotopes . The element's atomic mass in atomic mass units is a weighted average mass of the element's isotopes. Sometimes a periodic table cites a single value for atomic weight.
What does the symbol for silver mean?
Usually, the symbol is an abbreviation of the element name, but some symbols refer to older names of the elements (for example, the symbol for silver is Ag, which refers to its old name, argentum) . The modern periodic table is organized in order of increasing atomic number. The atomic number is how many protons an atom of that element contains.
What is the valence of all elements in the first column?
For example, all of the elements in the first column are reactive metals that have a valence of +1. All elements in a row have the same outermost electron shell. A good periodic table is a great tool for solving chemistry problems. You can use an online periodic table or print your own.
Our Periodic Table of Elements Content
Here is a snapshot of the periodic table of elements we have created for you.
How to Read a Periodic Table of Elements
A periodic table of elements places and groups the elements which existence we have identified based on their nature. You may notice some gaps that the periodic table of elements has in the middle. That is because we place the transition metals group there which nature is quite different from other elements.
Elements Grouping and Classification in a Periodic Table of Elements
As you can see in one of our periodic table of elements legend, the table divides the elements into ten groups. They are the alkali metals, alkali earth metals, transition metals, post-transition metals, metalloids, nonmetals, halogens, noble gases, lanthanides, and actinides.
How to Create the Table in Excel
Want to learn how to create a periodic table of elements in excel? It is very simple and you should be able to do it if you have mastered the basic functions in excel!
How to Modify this Table in Its Excel Template
Want to modify the display of our periodic table of elements in its excel file? You should be able to do most things we did to create the table beforehand!
Additional Note
To retrieve the information about an element from our periodic table in excel, you can use the HLOOKUP or INDEX MATCH. You may want to create a separate table first though that contains all the elements information you need. This is so you can retrieve the information you need later much easier.