How do you calculate valence?
How do you calculate valence electron concentration? To calculate the valence-electron contribution of an element one has to subtract 8 from the total number of outer-shell electrons er which is equal to the group number of the transition element in the periodic system.For example Pd with er= 10 provides only 2 valence electrons.
How do you find the number of valence electrons?
Part 2 Part 2 of 2: Finding Valence Electrons With an Electron Configuration
- Learn how to read an electron configuration. Another way to find an element's valence electrons is with something called an electron configuration.
- Find the electron configuration for the element you are examining. ...
- Assign electrons to orbital shells with the Octet Rule. ...
- Find the number of electrons in the outermost shell. ...
Is there an easy way to find number of valence electrons"?
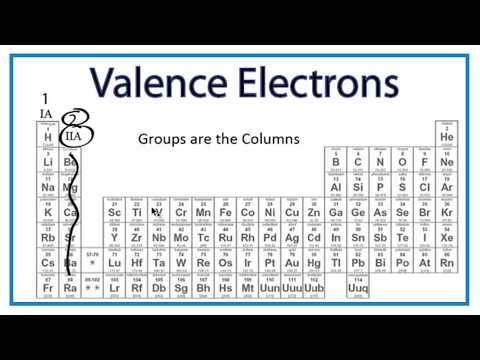
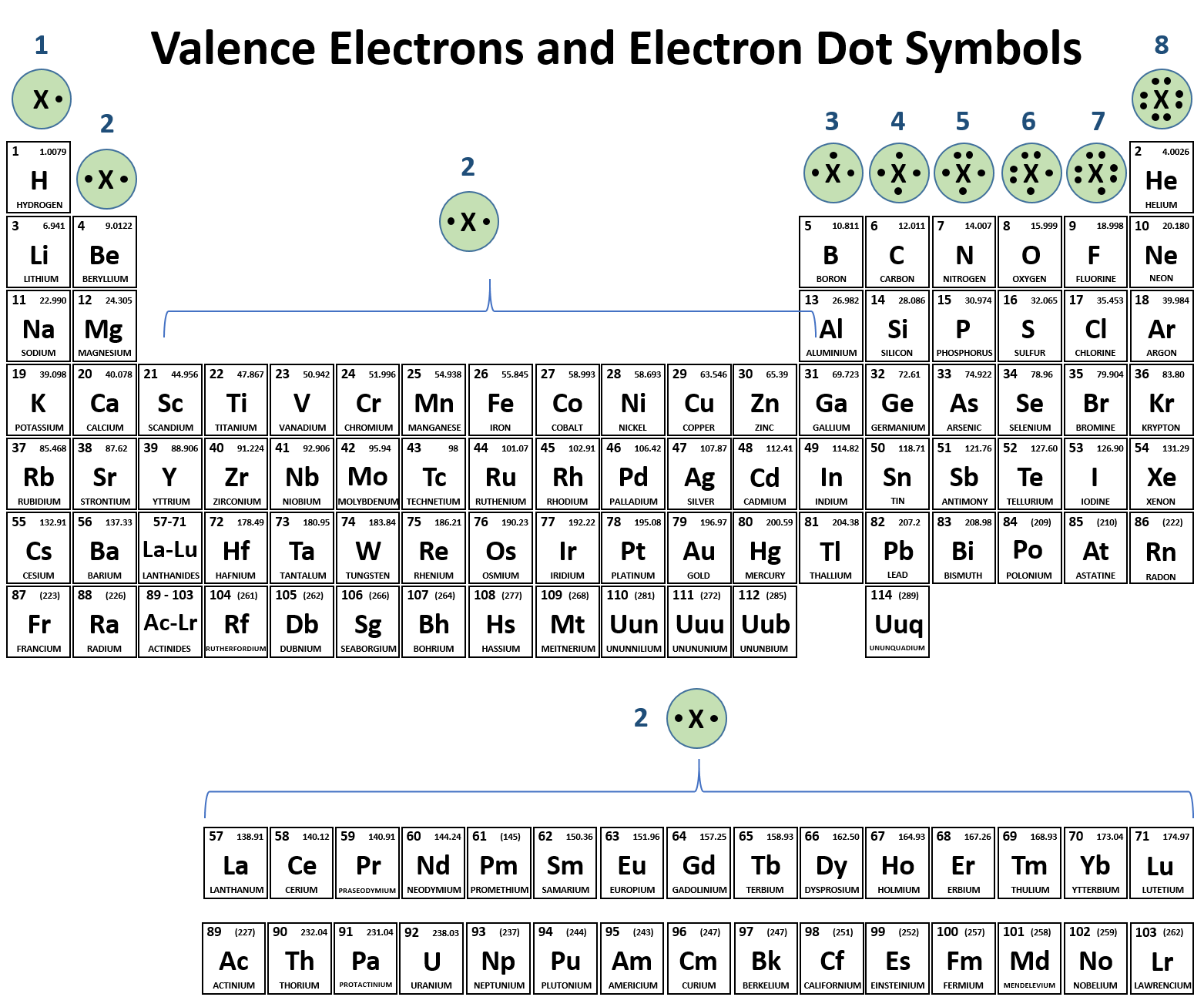
To find valence electrons using a period table , first see if your atom is a transitional metal, which are the elements in the middle rectangle of the table. If the atom is outside this block, locate its group number along the top of the table. The ones digit in the group number is the number of valence electrons.
How do you know how many valence electrons are in an element?
In order to determine the number of valence electrons of an element, we only have to refer to the periodic table and search for the position of the element in it . The periodic table is a clean arrangement of all elements discovered so far.
How to find valence electrons in a period table?
To find valence electrons using a period table, first see if your atom is a transitional metal, which are the elements in the middle rectangle of the table. If the atom is outside this block, locate its group number along the top of the table. The ones digit in the group number is the number of valence electrons.
How to determine the number of valence electrons?
Determine the number of valence electrons based on the group number. Once again, the group number of the element you are examining can tell you its valence electrons. However, for the transition metals, there isn't a pattern you can follow — group number will usually correspond to a range of possible numbers of valence electrons. These are:
How to find the number of valence electrons in an atom?
Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones place of the group number is the number of valence electrons in an atom of these elements. In other words:
How many electrons does sodium have?
So, for our example, we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital. That's 11 electrons total — sodium is element number 11, so this makes sense.
How many valence electrons does carbon have?
In our example, since carbon is in group 14, we can say that one atom of carbon has four valence electrons.
How to label a column on the periodic table?
Label each column on the periodic table of elements from 1 to 18. Generally, on a periodic table, all of the elements in a single vertical column will have the same number of valence electrons. If your periodic table doesn't already have each column numbered, give each a number starting with 1 for the far left end and 18 for the far right end. In scientific terms, these columns are called the element "groups."
How many orbital shells does Selenium have?
For example, we know the element selenium has four orbital shells because it is in the fourth period. Since it is the sixth element from the left in the fourth period (ignoring the transition metals), we know that the outer fourth shell has six electrons, and, thus, that Selenium has six valence electrons.
How many electrons does magnesium have?
The magnesium element has 2 electrons in outermost orbit.
What are the two bottom rows on the periodic table?
Also the two bottom rows at the bottom of the periodic table are the inner transition elements (or f-block elements) also have the similar case. The inner transition elements have incomplete f-subshells and they are very close to the outer s-subshell. Hence, for inner transition elements, the electrons of both f-subshells as well as s-subshell ...
What is the highest quantum number?
Here, you can see that the highest principal quantum number is 3, and the total electrons in this principal quantum number is 2.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Do inner transition elements have valence electrons?
The inner transition elements have incomplete f-subshells and they are very close to the outer s-subshell. Hence, for inner transition elements, the electrons of both f-subshells as well as s-subshell behave like valence electrons. In some inner transition metals, the electrons of incomplete d-orbitals are also considered as valence electrons.