What are alkaline metals on the periodic table?
alkaline-earth metal, any of the six chemical elements that comprise Group 2 (IIa) of the periodic table. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
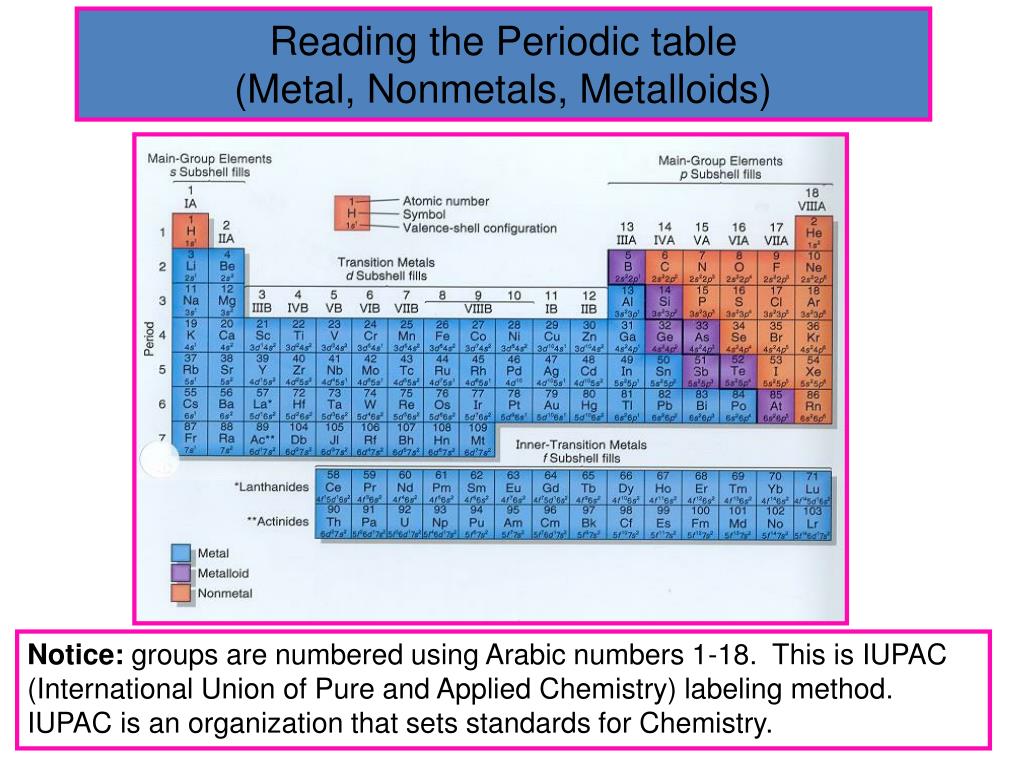
Where are inner transition metals located on periodic table?
Inner transition metals reside at the bottom of the periodic table. These elements are also known as rare earth elements since their natural occurrence is very low.
Which elements are metals on periodic table?
Metals: List of Elements
- Alkali Metals. Alkali metals are in group IA on the far left side of the periodic table. ...
- Alkaline Earth Metals. The alkaline earth metals are found in group IIA of the periodic table, which is the second column of elements.
- Basic Metals. ...
- Transition Metals. ...
- More About Metals. ...
What are the rare earth metals on the periodic table?
Rare earth metals are 17 elements present in the periodic table, they are known as 15 lanthanides, scandium, and yttrium. Metals come from the Earth's crust.
Is aluminium a metal on the periodic table?
Aluminium is a silvery-white, lightweight metal. It is soft and malleable. Aluminium is used in a huge variety of products including cans, foils, kitchen utensils, window frames, beer kegs and aeroplane parts.
Why is aluminum not a metal?
Aluminum is the most abundant metal on Earth, and one of the cheapest to buy. But it used to be more valuable than gold. Aluminum is the third most common element in the Earth's crust, but it also bonds easily with other elements. That means it is not found in nature as a pure metal.
What metal group is aluminum in?
Group 3AGroup 3A. Group 3A (or IIIA) of the periodic table includes the metalloid boron (B), as well as the metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). Boron forms mostly covalent bonds, while the other elements in Group 3A form mostly ionic bonds.
Is aluminum a metal yes or no?
Aluminium is a silvery-white metal, the 13 element in the periodic table. One surprising fact about aluminium is that it's the most widespread metal on Earth, making up more than 8% of the Earth's core mass. It's also the third most common chemical element on our planet after oxygen and silicon.
Why aluminium is a metal?
Aluminum has an atomic number and is a good heat and electrical conductor. In nature, it is hard, ductile, malleable, and lustrous with a high melting and boiling point. Hence it is considered as a metal.
Which groups are metals?
The metals found in Groups 1, 2, and 13–15 of the periodic table are referred to as main-group metals. Sometimes even group 12 is considered as main group metal.
How do you know if an element is a metal or nonmetal?
The metals are to the left of the line (except for hydrogen, which is a nonmetal), the nonmetals are to the right of the line, and the elements immediately adjacent to the line are the metalloids. When elements combine to form compounds, there are two major types of bonding that can result.
Which of the following is a metal?
Answer: Sodium is a metal.
What is the difference between metal and aluminum?
Aluminium has a light weight compared to other metals. It is malleable, i.e, it can be deformed under pressure. These properties of aluminium has made it to be used in aircraft manufacturing.
Why is aluminum not a metalloid?
Explanation: Aluminum is a ductile and malleable metal. Although it has some unusual properties that might make it seem metalloid, it is not a metalloid because, on the periodic table, it lies along the line that differentiates between metals, nonmetals, and metalloids.
Is aluminium a strong metal?
Many aluminum grades can be considered of very high strength, even comparable to some steels. Nevertheless, comparing samples of the same size of the strongest aluminum alloys and steel alloys, more often than not, steel will be the strongest.
Does aluminum break easily?
Aluminum is much more malleable than steel, meaning it can be successfully bent or extruded into a variety of custom shapes or profiles without suffering breaks or cracks. Aluminum is also known to be very ductile, enabling it to be stretched without breaking.
Aluminum in Periodic table
Aluminum element is in group 13 and period 3 of the Periodic table. Aluminum is the p-block element and it belongs to boron group.
Properties of Aluminum
The physical and chemical properties of aluminum element are mentioned below.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
Where Is Aluminum Found On The Periodic Table?
Aluminum is the 13th element on the periodic table. It is located in period 3 and group 13.
Aluminum Facts
Aluminum is element number 13 with the element symbol Al. Under ordinary pressures and temperatures, it is a light shiny silver solid metal.
What are the characteristics of aluminium?
Chemical Characteristics. Aluminium is a reactive metal. It is readily oxidized when exposed to air. The most common oxidation state of Aluminium is +3. Aluminium reacts with oxygen and sulfur to form oxides and sulfates, which have various industrial applications. It readily reacts with acids and bases.
Where is aluminium found?
It primarily exists in form of ores and the most common ore of Aluminium is bauxite. The largest producers of Aluminium in the world are China, Russia, Bahrain, and South Africa.
What is aluminium foil used for?
Aluminium foils are widely used all over the world. Aluminium is used in the manufacturing of cars, trucks, bicycles and marine vessels. Aluminium is widely used construction and building material, such as doors, building wire, windows and roofing.
How many isotopes of aluminum are there?
Isotopes of Aluminium. Aluminium has only one stable isotope, Aluminium-27. Among the radioactive isotopes, the most stable is Aluminium-26 and have a half life of about 720,000 years.
When was aluminum discovered?
Aluminium, or Aluminum in American spelling, is an ancient metal and has a diverse range of uses. It was discovered in 1824 and its light weight, high electrical conductivity and high resistant to corrosion has make it an industrially attractive metal.
Does aluminum affect soil fertility?
Inhalation of powdered welding fumes of Aluminium can lead to fibrosis of the lungs [4]. In environment, Aluminium can affect the soil fertility as it reacts with phosphate and makes it less available for the plants [5].
Is aluminum a toxic substance?
Aluminium is not toxic. In most cases, it is well tolerated if ingested or inhaled by humans or plants. However, very rare evidence of Aluminium toxicity is related to vitamin D deficiency, anemia and renal damage. Inhalation of powdered welding fumes of Aluminium can lead to fibrosis of the lungs [4].
What is the symbol for aluminum?
Aluminium is a light weight metal with silvery white appearance [1]. It is denoted by symbol “Al”. In Purest form, the aluminium metal is bluish- white in colour.
Where did aluminum get its name?
Aluminum is given this name from a word of Latin origin “Alumen or alum”. Guyton de Morveau gave it name “alumina” in 1761. After that, it was given name “Alumium” by Humphrey Davy in 1807. After some time, he renamed it as “Aluminum” [10]. After some time conforming with named of a lot of other elements, it was renamed as Aluminium. This name was further adopted by IUPAC and is still used all over the world except in North America. So both spellings “Aluminum” and “Aluminium” are considered correct in periodic table.
What is the substance having greater amount of naturally occurring Alumnium and impurities present in bauxite?
Bauxite is the substance having greater amount of naturally occurring Alumnium and impurities present in bauxite can be removed from aluminium. 90 % of bauxite is used for aluminium production. Bauxite is hydrated oxide of aluminium. Impurities present in bauxite are silica, ferric oxide and clay.
What is the purpose of an alloy?
When any element or group of elements are added to Alumnium in order to improve the physical properties , then they are known as alloys of Alumnium. Aluminium is usually used in alloy form as pure aluminium is not strong. For this purpose, some alloys are produced to improve the strength of aluminium products but keeping in mind that light weight nature of metal remains same. Some alloys are shown in table below:
Why is aluminium used in marine applications?
This is due to the fact, aluminium has higher corrosion resistance. So, for marine travel, this quality is of much importance, along with being light in weight [19].
Why is aluminium used in cars?
From last many years, aluminium has been used in automotive industries. Major applications in this field includes: Auto body, chassis and other structural components. Light weight vehicles made up of aluminium parts, with maximum corrosion resistance can help reducing fuel consumption. It is easier to clean this metal and that’s reason it is used in automotive industry. No surface protection is required if aluminium is used.
What is the third most abundant metal in the Earth's crust?
Aluminium is third most occurred metal present in earth crust. It covers almost 7% to 8 % of earth’s crust [ 7] [8].
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N. Nitrogen is a colourless, odourless unreactive gas that forms about 78% of the earth’s atmosphere.
How many electrons are in neon?
Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. The chemical symbol for Neon is Ne. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. 20.1797 amu.
What is the atomic number of beryllium?
Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structure. The chemical symbol for Beryllium is Be. Beryllium is a hard, grayish metal naturally found in mineral rocks, coal, soil, and volcanic dust.
What is the symbol for Copernicium?
The chemical symbol for Copernicium is Cn. Nihonium is a chemical element with atomic number 113 which means there are 113 protons and 113 electrons in the atomic structure. The chemical symbol for Nihonium is Nh.
What is the chemical symbol for Darmstadtium?
The chemical symbol for Darmstadtium is Ds. Roentgenium is a chemical element with atomic number 111 which means there are 111 protons and 111 electrons in the atomic structure. The chemical symbol for Roentgenium is Rg.
Which element has the same electron configuration in the outer electron shell?
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How are atoms determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
What is the most common type of boron?
There are over 100 different borate minerals, but the most common are: borax , kernite, ulexite etc. Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B.
What is the most abundant element in the Earth's crust?
Aluminium is a silvery-white, soft, nonmagnetic, ductile metal in the boron group. By mass, aluminium makes up about 8% of the Earth’s crust; it is the third most abundant element after oxygen and silicon and the most abundant metal in the crust, though it is less common in the mantle below.
Where is beryllium found?
Beryllium is a hard, grayish metal naturally found in mineral rocks, coal, soil, and volcanic dust . The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times because of the toxicity of inhaled beryllium-containing dusts that can cause a chronic life-threatening allergic disease in some people called berylliosis.
What are metals in Periodic table?
Metals are the elements which have the tendency to donate or lose electrons to form positive ions.
Where are metals located on the periodic table?
The metals are located on the left side of the Periodic Table.
How many electrons do metals lose in a chemical reaction?
The atoms or metals have generally 1, 2 or 3 electrons in the outermost orbit, and they lose these electrons during a chemical reaction.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Which group of metals is the most reactive?
They are the Alkali metals of group 1. In 1st group, as we move down from top to bottom, the reactive of metals increases. Thus the bottom most element of group 1 (i.e francium) is the most reactive metal on the Periodic table. ( Note: Francium is a laboratory made element.
Why do metals make a ringing sound?
Metals produce ringing sound when they are stuck hard. This indicates that metals are sonorous in nature.
What are the elements in group 3 to group 12?
The elements lying in group 3 to group 12 are known as Transition metals (or transition elements). Transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14. Thus these metals are known as “Transition metals”.
