
Key Concepts
- The electrons surrounding an atom are located in regions around the nucleus called “energy levels”.
- An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be.
- The first energy level is closest to the nucleus. ...
- Each energy level can accommodate or “hold” a different number of electrons before additional electrons begin to go into the next level.
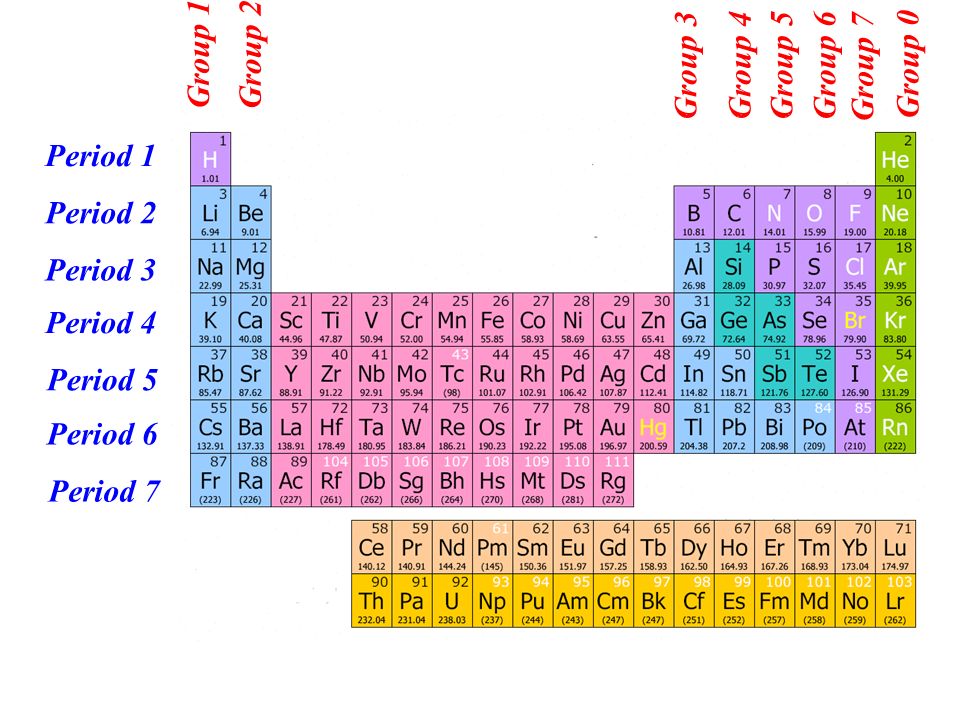
How many principal energy levels are on the periodic table?
•There are 7 energy levels write these on your periodic table.(they correspond to the horizontal rows known as periods.) •There are at least 4 possible types of sublevels—given labels: s, p, d, or f Periodic Table write the levels 1-7 on your periodic table 1 2 3 4 5 6 7
Are elements organized in periods by energy level?
Elements are organized by period and group, with the period corresponding to the principle energy level, and the group relating to the extent the subshells are filled. The properties of an atom relate directly to the number of electrons in various orbitals, and the periodic table is much like a road map among those orbitals such that chemical ...
What is the heaviest element in the periodic table?
What is the heaviest elements in order?
- Plutonium 244 u. Not only is plutonium very dense, but it has a very high atomic weight as well.
- Uranium 238.028 u. …
- Thorium 232.037 u. …
- Protactinium 231.0359 u. …
- Actinium 227 u. …
- Radium 226 u. …
- Francium 223 u. …
- Astatine 210 u. …
What is the sixteenth element in the periodic table?
Sulfur is the 16th element of the periodic table of elements. [ ] indicates the atomic weight of the longest-lived isotope. * indicates the IUPAC conventional atomic weights; standard atomic weights for these elements are expressed in intervals; see iupac.org for an explanation and values.
Where is the energy level on a periodic table?
The highest energy level number (1 through 7) for the electrons in an atom corresponds to the period (or row) in the periodic table to which that atom belongs. Because there are 7 periods in the table, there are 7 energy levels. For example, hydrogen (H) is in the first period, so it has only one energy level.
What are the energy levels of an atom?
The first energy level is also called level 'K'. The second level is called level L, third energy level as M, and so on. Electrons in the outermost energy level are also called Valence electrons. Various properties of atoms are based on these valence electrons.
What are the 4 main energy levels?
There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Within each shell of an atom there are some combinations of orbitals.
Which elements has 7 energy levels?
ElementElement NumberNumber of Electrons in each LevelCarbon64Nitrogen75Oxygen86Fluorine9751 more rows
What is meant by energy level?
energy level, also called energy state, in physics, any discrete value from a set of values of total energy for a subatomic particle confined by a force to a limited space or for a system of such particles, such as an atom or a nucleus.
What is energy levels of electrons?
Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. As you go farther from the nucleus, electrons at higher energy levels have more energy. An orbital is a volume of space within an energy level where an electron is most likely to be found.
How many main energy levels are there?
Theoretically there are an infinite number principal energy levels and sublevels. If you are just starting to study chemistry, you should only be concerned with the first 4 sublevels. Each sublevel is assigned a letter. The four you need to know are s (sharp), p (principle), d (diffuse), and f (fine or fundamental).
How do you find the energy level of valence electrons?
0:4811:32Valence Electrons and the Periodic Table - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe valence electrons are the electrons in the outermost energy level so therefore a nitrogen hasMoreThe valence electrons are the electrons in the outermost energy level so therefore a nitrogen has five valence electrons so here are the five valence electrons of the nitrogen atom.
How are electrons arranged in energy levels?
The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals.
How do you calculate energy level?
where 13.6 eV is the lowest possible energy of a hydrogen electron E(1)....Summary.Value of the Atomic Radiusr ( n ) = n 2 × r ( 1 )The value of the energy emitted for a specific transition is given by the equationh v = Δ E = ( 1 n l o w 2 − 1 n h i g h 2 ) 13.6 e VThe formula for defining energy levelE = E 0 n 21 more row
Which element has 4 energy levels and 8 valence electrons?
Calcium is an element.
What element has 4 energy levels and 2 valence electrons?
CalciumMeans Calcium is an element which lies at Period 4 in Periodic table and having two valence electrons (Period 4 means 4th shell being occupied having 2 electrons in 4th or outermost shell)
How do you calculate the energy level of an atom?
0:1616:26How to Calculate Energy Level Transitions for the Hydrogen Atom ...YouTubeStart of suggested clipEnd of suggested clipSo for hydrogen. The energy of any of these energy levels is equal to this constant here divided byMoreSo for hydrogen. The energy of any of these energy levels is equal to this constant here divided by n squared. And n stands for the energy.
Why do atoms have different energy levels?
Transitions among the various orbitals are unique for each element because the energy levels are uniquely determined by the protons and neutrons in the nucleus. We know that different elements have different numbers of protons and neutrons in their nuclei.
Where is the highest energy level in an atom?
valence shellEach atom has different energy shells or electrons, and the shell with the highest energy is called the valence shell. Electrons contained in the valence shell are called valence electrons.
How do you determine the number of energy levels?
The number of electrons in each energy level is displayed on the periodic table. The number of elements in each row shows how many electrons it takes to fill each level. Hydrogen and helium are in the first row, or period, on the periodic table. Therefore, the first energy level can have a total of two electrons.
What is the energy level of a nucleus?
An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be.
What is the fourth energy level?
Fourth energy level = 1 , 2. Read more about the periodic table in the teacher background section. A certain number of electrons go into a level before the next level can have electrons in it. After the first energy level contains 2 electrons (helium), the next electrons go into the second energy level.
What does the nucleus represent in the energy level model?
Tell students that this energy level model represents an atom. The nucleus is represented by a dot in the center, which contains both protons and neutrons. The smaller dots surrounding the nucleus represent electrons in the energy levels.
How many electrons does neon have?
Explain that neon has 10 protons and 10 electrons. There are 2 electrons on the first energy level and 8 electrons on the second level. Beryllium–fluorine. Help students fill in the correct number of electrons in the energy levels for the rest of the atoms in period 2.
Why are electrons shown in pairs in energy level diagrams?
The pairing of electrons is meant to represent that electrons are in separate orbitals within each energy level . At the middle school level, it is not necessary for students to learn about electron orbitals. This information is offered so that it is clearer to you why electrons are often shown in pairs in energy level diagrams and in the dot diagrams used as an extension at the end of this chapter. An orbital defines a region within an energy level where there is a high probability of finding a pair of electrons. There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level.
How many protons does argon have?
Argon. Explain that argon has 18 protons and 18 electrons. There are 2 electrons on the first energy level, 8 electrons on the second level, and 8 electrons on the third energy level. Have students complete the energy level model for argon in their periodic table.
What is the name of the column in the periodic table that atoms in the same column share certain characteristics?
Tell students that in the periodic table atoms in the same column, called a group, share certain characteristics and can react in a similar way.
What is the energy level of a nucleus?
An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be.
What is the fourth energy level?
Fourth energy level = 1 , 2. Read more about the periodic table in the teacher background section. A certain number of electrons go into a level before the next level can have electrons in it. After the first energy level contains 2 electrons (helium), the next electrons go into the second energy level.
What does the nucleus represent in the energy level model?
Tell students that this energy level model represents an atom. The nucleus is represented by a dot in the center, which contains both protons and neutrons. The smaller dots surrounding the nucleus represent electrons in the energy levels.
Why are electrons shown in pairs in energy level diagrams?
The pairing of electrons is meant to represent that electrons are in separate orbitals within each energy level . At the middle school level, it is not necessary for students to learn about electron orbitals. This information is offered so that it is clearer to you why electrons are often shown in pairs in energy level diagrams and in the dot diagrams used as an extension at the end of this chapter. An orbital defines a region within an energy level where there is a high probability of finding a pair of electrons. There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level.
How many electrons are in the second energy level of sodium?
Explain that the second energy level can only have 8 electrons so the next electron in sodium has to be on the next (third) level. Argon. Explain that argon has 18 protons and 18 electrons. There are 2 electrons on the first energy level, 8 electrons on the second level, and 8 electrons on the third energy level.
How many protons does lithium have?
Explain that lithium has 3 protons and 3 electrons. There are 2 electrons on the first energy level and 1 electron on the second. Explain that the first energy level can only have 2 electrons so the next electron in lithium is on the next (second) level.
Which electrons are in the energy level farthest from the nucleus?
The electrons in the energy level farthest from the nucleus are called valence electro ns. Atoms in the same column (group) in the periodic table have the same number of valence electrons .
Which element goes into the first energy level?
1st Period: Hydrogen and helium —Electrons go into the first energy level. After the first level has two electrons, the next electron goes into the second level. 2nd Period: Lithium to neon—Electrons go into the second level. After the second level has 8 electrons,the next electron goes into the third level. 3rd Period: Sodium to argon—Electrons go ...
Where are electrons located in the energy level cross section?
Energy Level Cross Section. Electrons are in regions around the nucleus that are different distances away from the nucleus. The electrons surround the nucleus in 3 dimensions but it is easier to show an energy level model in two dimensions like the model that looks like a target. Oxygen Atom.
How many protons does oxygen have?
Oxygen Atom. This energy level model shows two electrons on the first energy level and six electrons on the second energy level. Since this atom has a total of eight electrons, it also has eight protons. The atom with eight protons in its nucleus (atomic number 8) is oxygen. Periodic Table of Energy Levels.
What atom has 8 protons?
The atom with eight protons in its nucleus (atomic number 8) is oxygen . Periodic Table of Energy Levels. Please click on the image above to see a larger version. Each energy level holds a certain number of electrons before electrons go into the next level.
What happens after the second level of a molecule has 8 electrons?
After the second level has 8 electrons,the next electron goes into the third level. 3rd Period: Sodium to argon—Electrons go into the third energy level. After the third energy level has 8 electrons, the next electron goes into the fourth level.
Is the reaction between calcium and the acids as hot as between sodium and potassium?
The reaction between calcium and the acids does not get as hot as between sodium or potassium with the acids. The demonstration shown in this video is very dangerous. Do not attempt to perform this demonstration.
How many electrons are in the outermost energy level?
8 electrons in the outermost energy level
What is the atomic number of an element?
The atomic number is the number of protons in the element
What are the protons and neutrons in the nucleus called?
The protons and neutrons in the nucleus collectively are called nucleons since both are located in the nucleus
What happens when atoms have the same number of protons but different number of neutrons?
Atoms with the same number of protons but different number of neutrons which result in different mass numbers
What is periodic table?
The periodic table is a chart displaying information about the elements. Elements are arranged in the table in a specific pattern that helps to predict their properties and to show their similarities and differences.
Why is the periodic table called the periodic table?
In the periodic table, the periods are the horizontal rows that extend from left to right . These periods consist of as few as two elements and as many as thirty-two elements.
How to determine the electron configuration of an element?
The electron configurations of elements may be better understood by dividing the periodic table into s orbital, p orbital, d orbital, and f orbital blocks. The width of each orbital block is related to the maximum number of electrons that can be held by a particular orbital (i.e., s orbitals can hold up to two electrons, so the s block is two elements wide; p orbitals can hold up to six electrons, so the p block is six elements wide, etc.).
What are the two main classes of elements?
Two main classes of elements are metals and nonmetals.
How are atoms arranged in the periodic table?
Atoms are arranged in the periodic table of the elements according to their properties. For example, metals are grouped together, and nonmetals (except for hydrogen) are grouped together. Elements have greater metallic character moving from right to left and from top to bottom within the periodic table. The stairstep line that begins between boron (B) and aluminum (Al) and moves down and right to polonium (Po) and astatine (At) is the dividing line between metals and nonmetals. The elements bordering the stairstep line have properties between those of metals and nonmetals, so these elements are called metalloids. This division is shown by the different colors in the periodic table below.
What is the division of elements into vertical groups by column?
The division of elements into vertical groups by column creates families of elements. Elements in the same group all have the same number of valence electrons and therefore similar chemical properties.
