What is group vs period?
Summary
- The periodic table is arranged in order of atomic number
- A period is a horizontal row of the periodic table.
- A group is a vertical row of the periodic table.
What is a group or family on the periodic table?
What is a family on the periodic table?
- Team 1: antacids steels, or lithium family.
- Team 2: alkaline planet steels, or beryllium family.
- Team 3: the scandium family.
- Team 4: the titanium family.
- Team 5: the vanadium family.
- Team 6: the chromium family.
- Team 7: the manganese family.
- Team 8: the iron family.
What are the groups and families on the periodic table?
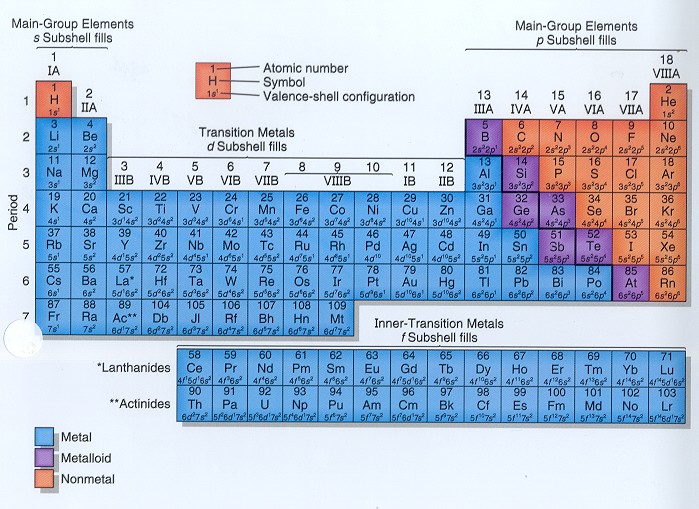
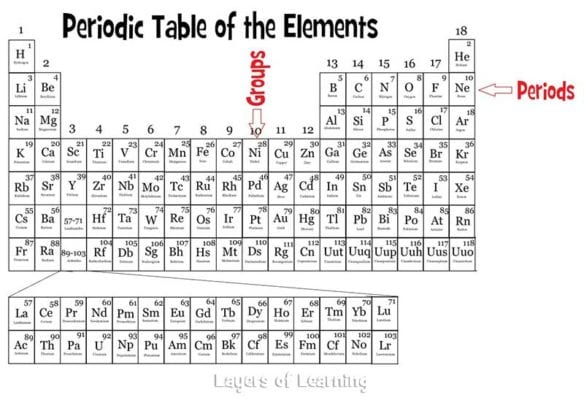
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods. Group (family): A vertical column in the periodic table.
What is a period or group?
Period Group; Direction: Periods are the horizontal rows on the modern periodic table: Groups are the vertical columns that runs through the top on the periodic table: Properties: The elements in a period do not have similar properties. The elements in each group have some similar properties, but not entirely identical properties. Similarity

What do groups and periods mean on a periodic table?
Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. Families: Elements that have the same number of valence electrons and therefore similar properties.
What are periods on a periodic table?
The horizontal rows of the periodic table are called periods. Each period corresponds to the successive occupation of the orbitals in a valence shell of the atom, with the long periods corresponding to the occupation of the orbitals of a d subshell.
How do you remember periods and groups?
0:292:53Periods & Groups In The Periodic Table | Properties of Matter - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach new period row represents a new shell elements in the first period have one shell and as we goMoreEach new period row represents a new shell elements in the first period have one shell and as we go down the shells.
How do you find the period and group of an element?
To determine the group, we need to understand some rules:If the element is in s block, then the group number is equal to the number of valence electrons. ... If the element is in the p block, then the number of the group can be determined by the formula: (number of valence electrons + 10).for groups.More items...
What is period 7 on the periodic table?
The Actinide series contains elements with atomic numbers 89 to 103 and is the sixth period and third group of the periodic table. The series is the row below the Lanthanide series, which is located underneath the main body of the periodic table.
Are periods left and right?
The periods of the periodic table are the rows that run from left to right in the periodic table. The elements are not arranged in these because of similar properties but because of their increasing atomic number from left to right. Periods are the rows of the periodic table.
What is group and period in periodic table 10?
The vertical columns in the periodic table are called 'groups' and horizontal rows in the table are called periods.
What element is in period 2?
The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon.
What are groups and periods?
Groups and Periods are the columns and rows of the periodic table. In this section you will explore how the periodic table was put together and the two main arrangement of the periodic table focusing on the groups and periods and metals and non metals.
What is the period of the periodic table?
The periods of the table are the rows that go from left to right. As you move from left to right there are no similarities in these elements unlike in the groups but the number of protons and neutrons increases as the masses of the elements get larger. Mendeleev also arranged his periodic table using this idea, as you go across the atoms get larger and as you go down they get larger.
How did Mendeleev create the periodic table?
When Mendeleev created the periodic table he arranged the elements he knew of into groups based on their properties and similarities. He then put the groups next to each other and he found that as he went across each of the groups he worked out the mass of them increased each time.
Why are groups given a number?
Groups are given a number to show where they are in the periodic table and also to identify the group of elements in them . Mendeleev put elements with similar properties and that react in similar ways into the same groups.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
Why is period 1 the shortest period?
Period 1 of the periodic table is given the name shortest period because there are only two elements in period 1.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
What is the difference between periods and groups?
Updated August 10, 2019. Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
How many periods are there for naturally occurring elements?
There are seven periods for naturally occurring elements :
What are the common properties of elements in a group?
Elements in a group share a common number of valence electrons. For example, all of the elements in the alkaline earth group have a valence of two. Elements belonging to a group typically share several common properties.
Why are there more periods than others?
There are more elements in some periods than others because the number of elements is determined by the number of electrons allowed in each energy sub-level. There are seven periods for naturally occurring elements :
Is hydrogen a metal?
Within this classification system, hydrogen is a nonmetal. The nonmetals, halogens, and noble gases are all types of nonmetallic elements. The metalloids have intermediate properties. All of the other elements are metallic.
How many periods are there in the periodic table?
Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases.
How are elements organized in the periodic table?
The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the same period have the same number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. From this deduction, he formed the periodic table. It is important to note how the location of elements on this table tells us about their properties. A quick way to understand an element’s chemical and physical properties is to know the periodic trends. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. For a more in-depth explanation of periodic trends, click here.
What group are alkali metals in?
The Alkali Metals (Group 1) The alkali metals consist of all of the elements in group one with the exception of hydrogen. These elements are extremely reactive and for this reason, are usually found in compounds. In addition, they are water-sensitive (they react violently with water), so they must be stored in oil.
What are noble gases?
The noble gases, also called aerogens, are inert gases. Some examples include argon, krypton, and neon. They can be found in group eighteen on the periodic table. Likewise, this means they have a complete valence shell. For this reason, they are stable and relatively unreactive.
What is an element in the periodic table?
Vocabulary. Elements: A pure substance composed of a single atom. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. Families: Elements that have the same number ...
What is the name of the group of elements that are found in the three states of matter at standard temperature?
The name halogen means “salt formers” in greek. This is evident in nature as halogens interact with metals to form various salts. On another note, the halogens are a unique group of elements. They are the only periodic family that contains elements in the three states of matter at standard temperature. There are 6 halogens and they are located in group 17. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are highly reactive, highly electronegative, and highly toxic non-metals.
Which metals are the second most reactive?
The alkaline earth metals are the second most reactive family on the periodic table (following behind the alkali metals). Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. They are also good thermal and electrical conductors.
How does the periodic table organize the elements?
The classic Periodic Table organizes the chemical elements according to the number of protons that each has in its atomic nucleus. (Image credit: Karl Tate, Livescience.com contributor)
Which group of the periodic table is alkaline earth metals in?
Alkaline-earth metals: The alkaline-earth metals make up Group 2 of the periodic table, from beryllium (Be) through radium (Ra). Each of these elements has two electrons in its outermost energy level, which makes the alkaline earths reactive enough that they're rarely found alone in nature. But they're not as reactive as the alkali metals. Their chemical reactions typically occur more slowly and produce less heat compared to the alkali metals.
What are the groups of metals that are radioactive?
All are radioactive. The actinides and the lanthanides together form a group called the inner transition metals. Transition metals: Returning to the main body of the table, the remainder of Groups 3 through 12 represent the rest of the transition metals.
What are the elements in the actinides?
Actinides: The actinides line the bottom row of the island and comprise elements 89, actinium (Ac), through 103, lawrencium (Lr). Of these elements, only thorium (Th) and uranium (U) occur naturally on Earth in substantial amounts. All are radioactive. The actinides and the lanthanides together form a group called the inner transition metals.
How many elements were there at the time of Mendeleev?
There were only about 60 elements known at the time, but Mendeleev realized that when the elements were organized by weight, certain types of elements occurred in regular intervals, or periods. Today, 150 years later, chemists officially recognize 118 elements (after the addition of four newcomers in 2016) and still use Mendeleev's periodic table ...
Why is the period of sodium longer?
Moving down the table, periods are longer because it takes more electrons to fill the larger and more complex outer levels. The columns of the table represent groups, or families, of elements.
Why is Mendeleev's table a circle?
Because of the cyclical nature created by the periodicity that gives the table its name, some chemists prefer to visualize Mendeleev's table as a circle.