What is the maximum number of columns allowed in a table?
For the columns in a table, there is a maximum limit of 1024 columns in a table. SQL Server does have a wide-table feature that allows a table to have up to 30,000 columns instead of 1024. SQL Server 2008 and up have a Sparse Columns feature that can optimize storage when you have many columns with NULL values in the rows of the table.
How to count columns in a table?
Steps to count columns in a table using MySQL in python
- import MySQL connector
- establish connection with the connector using connect ()
- create the cursor object using cursor () method
- create a query using the appropriate mysql statements
- execute the SQL query using execute () method
- close the connection
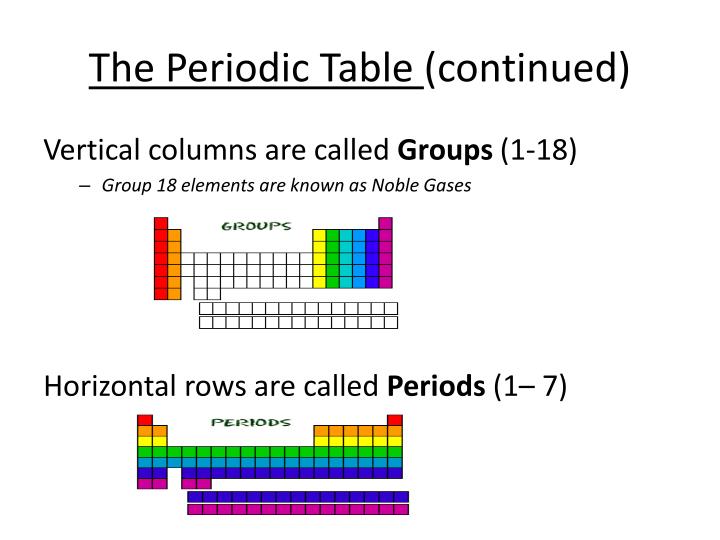
Which is a column running up and down in the periodic table?
By arranging the elements in this way, those with similar properties (characteristics) are grouped together. As with any grid, the periodic table has rows running left to right, and columns running up and down. The rows are called PERIODS and the columns are called GROUPS.
How many columns of elements does the periodic table contain?
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column. What are the columns up and down of a periodic table called? The vertical columns on the periodic table are called groups or families because of their similar chemical behavior.

What are the horizontal rows on the periodic table called?
Follow Us: The horizontal rows on the periodic table of the elements are called periods. Every element in a period has the same number of atomic orbitals. For instance, hydrogen and helium are in the first period, so they both have electrons in one orbital. The columns on the table divide the elements into groups with the same number ...
What are the electrons in the outer shell of an element called?
The columns on the table divide the elements into groups with the same number of electrons in their outer shells. These electrons, called valence electrons , cause them to share chemical properties.