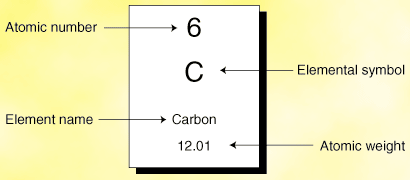
What are the row's and column's called in the periodic table?
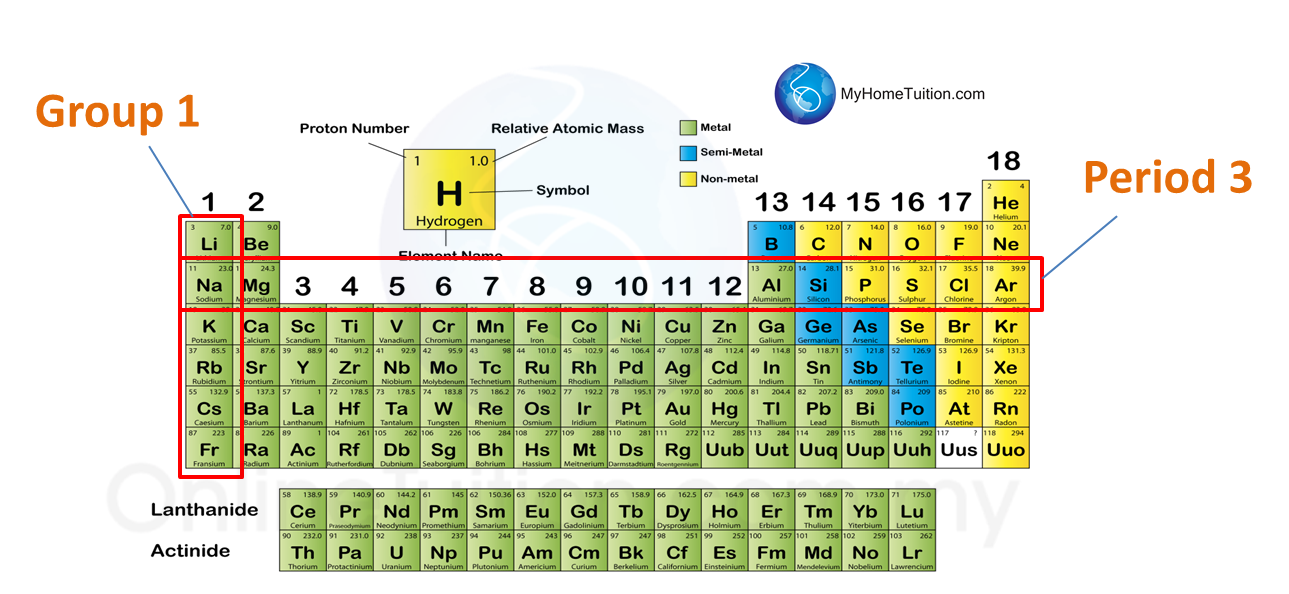
Summary The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods.
Which is a column running up and down in the periodic table?
By arranging the elements in this way, those with similar properties (characteristics) are grouped together. As with any grid, the periodic table has rows running left to right, and columns running up and down. The rows are called PERIODS and the columns are called GROUPS.
What do elements in the same column in the periodic table have in common?
Short answer they have the same amount of outermost electrons and hence similar chemical properties. Explanation: For the elements in column 1,2 and 13-18 the atoms in the same column have the same amount of outermost electrons, called valence electrons. Atoms in column 1 (H, Li and Na for example) have 1 valence electron.
How many columns of elements does the periodic table contain?
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column. What are the columns up and down of a periodic table called? The vertical columns on the periodic table are called groups or families because of their similar chemical behavior.
What are the columns called on a periodic table?
The periodic table is arranged by atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row (called a period) and column (called a group).
What are rows and columns in the periodic table?
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column.
What is the first column of the periodic table called?
Element in the first column of the periodic table are called alkali metals.
What is column 1 on the periodic table?
Group 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These are (except for hydrogen) soft, shiny, low-melting, highly reactive metals, which tarnish when exposed to air.
What are rows in the periodic table?
Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases.
What do the rows mean on a periodic table?
The rows on the periodic table are called periods. All the elements in a period have valence electrons in the same shell. The number of valence electrons increases from left to right in the period. When the shell is full, a new row is started and the process repeats.
How many rows and columns are in the periodic table?
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column.
What do you call the rows on the periodic table of elements?
The periodic table involves vertical columns and horizontal rows. The horizontal rows are referred as periods. The vertical columns are referred as columns.
What are the rows of the periodic table called?
The rows of the periodic table are called periods. All elements within a period share the same highest electron energy level.
What are the two rows of elements below the body of the periodic table called?
The two rows of elements below the body of the periodic table are metals. Specifically, they are a collection of transition metals that are called the lanthanides and actinides or the rare earth metals.
What is the line between metals and nonmetals called?
Metalloids (or Semimetals ) There is a zig-zag line toward the right side of the periodic table that acts as a sort of border between metals and nonmetals. Elements on either side of this line exhibit some properties of metals and some of the nonmetals. These elements are the metalloids, also called semimetals.
What are the properties of nonmetals?
The elements on the right-hand side of the periodic table are the nonmetals. Nonmetals properties are: 1 usually poor conductors of heat and electricity 2 often liquids or gases at room temperature and pressure 3 lack metallic luster 4 readily gain electrons (high electron affinity) 5 high ionization energy
What are the three categories of elements?
The three broad categories of elements are metals, nonmetals, and metalloids. Most elements are metals. Nonmetals are located on the righthand side of the periodic table. Metalloids have properties of both metals and nonmetals.
Why is the periodic table important?
To get the most out of the table, it helps to know the parts of the periodic table and how to use the chart to predict element properties.
Which element has the same number of valence electrons?
Atoms of elements within a group have the same number of valence electrons. These elements share many similar properties and tend to act the same way as each other in chemical reactions. The rows in the periodic table are called periods. Atoms of these elements all share the same highest electron energy level.
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Do elements of the same group have the same number of valence electrons?
In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons.