What are the names of the rows in the periodic table?
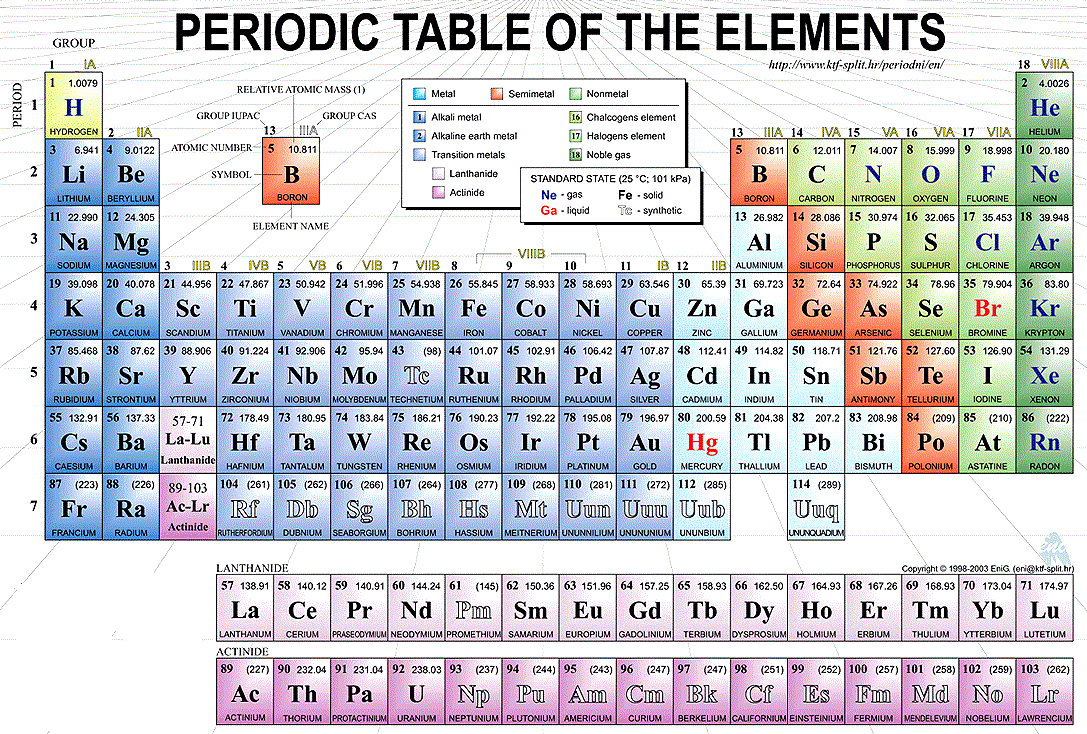
Key Takeaways: Parts of the Periodic Table The periodic table orders elements by increasing atomic number, which is the number of protons in the atom of an element. The rows of the periodic table are called periods. ... The columns of the periodic table are called groups. ... The three broad categories of elements are metals, nonmetals, and metalloids. ...
What do the rows mean on the periodic table?
What do periodic table rows mean? Each row represents one period; the period number of an element indicates how many of its energy levels house electrons. Sodium, for instance, sits in the third period, which means a sodium atom typically has electrons in the first three energy levels.
What happens along each row of the periodic table?
The word “periodic” means that within each row, or period, the elements show a pattern of characteristics. This is because the elements are listed in part by their electron configuration. Blocking in the periodic table: The periodic table can be broken into blocks, corresponding to the highest energy electrons.
What are the vertical columns of the periodic table called?
What Are the Vertical Columns on the Periodic Table? The vertical columns on the period table are called groups. There are 18 groups on the periodic table, and elements that are members of the same group share similar traits. All elements in the same group have the same number of electrons in their outer electronic shell.

What are the horizontal rows on the periodic table called?
Follow Us: The horizontal rows on the periodic table of the elements are called periods. Every element in a period has the same number of atomic orbitals. For instance, hydrogen and helium are in the first period, so they both have electrons in one orbital. The columns on the table divide the elements into groups with the same number ...
What are the electrons in the outer shell of an element called?
The columns on the table divide the elements into groups with the same number of electrons in their outer shells. These electrons, called valence electrons , cause them to share chemical properties.
