What are the horizontal lines on the periodic table called?
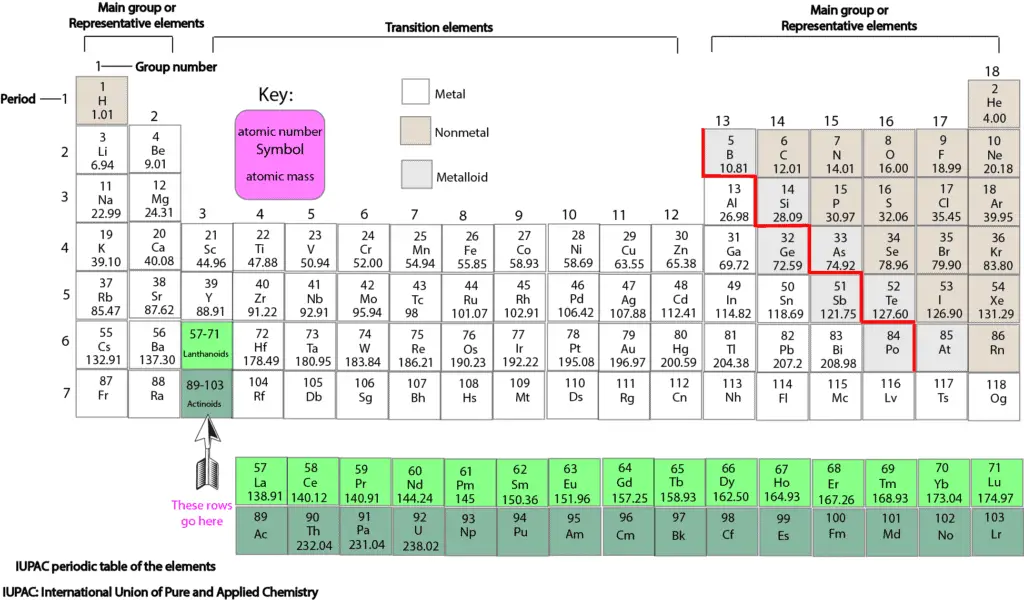
The horizontal rows are called period and the vertical columns are called groups. What element is soft and shiny? Group 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
What are the names of the columns and rows in periodic table?
Modern periodic table has 18 columns called groups and 7 rows called periods . Most of the elements in the periodic table are classified as. A group is a vertical column of the periodic table, based on the organization of the outer shell electrons. Mendeleev put elements with similar properties and that react in similar ways into the same.
How many columns are in a periodic table?
In the Modern periodic table, there are 18 columns and 7 rows.Columns are called groups and rows are known as periods. So, there are 18 groups and 7 periods in modern periodic table. There are 18 groups (columns) in the periodic table. Group 1 (also known as the alkali metals) is the column on the far left of the table.
Why is the periodic table arranged in columns?
Why are the elements on the periodic table grouped in columns the way they are? The columns of the table represent groups, or families, of elements.The elements in a group often look and behave similarly, because they have the same number of electrons in their outermost shell — the face they show to the world.
What are the columns of the periodic table called?
What are vertical columns?
About this website

What is a vertical column called?
Vertical columns in the periodic table are termed groups, whereas horizontal rows are termed periods.
What do you call the vertical rows in a periodic table?
The periodic table is composed of vertical columns called groups and horizontal rows called periods. The periodic table is divided into groups (vertical columns) and periods (horizontal rows).
What is vertical and horizontal in periodic table?
The horizontal rows are known as periods and vertical columns in the periodic table are known as groups. Solve any question of Classification Of Elements And Periodicity In Properties with:- Patterns of problems.
What are the horizontal and vertical rows called on the periodic table?
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column.
What are the horizontal rows called?
periodsThe horizontal rows are known as periods and vertical columns in the periodic table are known as groups.
What are rows and columns on the periodic table?
As with any grid, the periodic table has rows running left to right, and columns running up and down. The rows are called PERIODS and the columns are called GROUPS. Hydrogen (H) is the first element in the periodic table because it has just one proton in its nucleus.
What is a row in a periodic table?
When you look at the periodic table, each row is called a period (Get it? Like PERIODic table.). All of the elements in a period have the same number of atomic orbitals. For example, every element in the top row (the first period) has one orbital for its electrons.
What do you call the horizontal lines of the periodic table?
Elements that exhibit similar chemistry appear in vertical columns called groups (numbered 1–18 from left to right); the seven horizontal rows are called periods.
Vertical columns on the periodic table are called - Brainly.com
Click here 👆 to get an answer to your question ️ vertical columns on the periodic table are called _____ . The elements in each column have_____.
Matter Lesson 7: Pre-Quiz | Periodic Table Quiz - Quizizz
Q. Elements are arranged in the periodic table based on various patterns. For example, elements found in the rows near the top
Along each row of the periodic table, a. atomic mass increases from ...
Along each row of the periodic table, a. atomic mass increases from left to right. b. atomic mass decreases from left to right. c. atomic mass is the same for all elements in the row. d. atomic mass changes in no set pattern.
class 5
The Fish Tale Across the Wall Tenths and HundredthsParts and Whole Can you see the Pattern?
class 9
Circles Coordinate Geometry What is Democracy? Why Democracy?Nazism and the Rise of Hitler Socialism in Europe and the Russian Revolution
What are the columns of the periodic table called?
The columns of the periodic table are called groups and they contain elements with similar properties based primarily on the number of electrons in the outer shell. Are you sure there isn't another option?
What are vertical columns?
Vertical columns are groups. The elements in each column have similar properties,
What are the columns of the periodic table called?
The columns of the periodic table are called groups and they contain elements with similar properties based primarily on the number of electrons in the outer shell. Are you sure there isn't another option?
What are vertical columns?
Vertical columns are groups. The elements in each column have similar properties,
