What are groups and columns in the periodic table called?
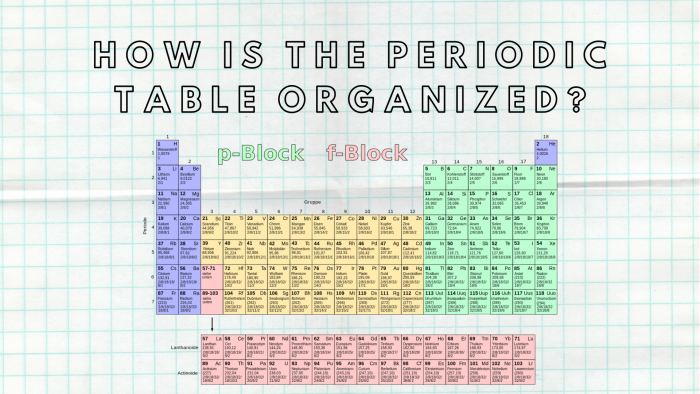
- The horizontal rows are called periods.
- The vertical columns are called groups.
- Elements in the same group are similar to each other.
- The metals are on the left and the non-metals are on the right (hydrogen is a non-metal but is often put in the middle).
What are the first 20 elements of the periodic table?
What are the first 20 elements in order?
- H – Hydrogen
- He – Helium
- Li – Lithium
- Be – Beryllium
- B – Boron
- C – Carbon
- N – Nitrogen
- O – Oxygen
- F – Fluorine
- Ne – Neon
What is 14 on the periodic table?
The carbon family is element group 14 of the periodic table. The carbon family consists of five elements: carbon, silicon, germanium, tin, and lead. It is likely that element 114, flerovium, will also behave in some respects as a member of the family.
What are the first 20 elements?
These are the first 20 elements, listed in order: H - Hydrogen He - Helium Li - Lithium Be - Beryllium B - Boron C - Carbon N - Nitrogen O - Oxygen F - Fluorine Ne - Neon Na - Sodium Mg - Magnesium Al - Aluminum Si - Silicon P - Phosphorus S - Sulfur Cl - Chlorine Ar - Argon K - Potassium Ca - ...

Where is the column on the periodic table?
As with any grid, the periodic table has rows running left to right, and columns running up and down. The rows are called PERIODS and the columns are called GROUPS.
What is called as the columns in the periodic table *?
The vertical columns of the periodic table are called groups.
What is the first column of the periodic table called?
Alkali metals: The alkali metals make up most of Group 1, the table's first column. Shiny and soft enough to cut with a knife, these metals start with lithium (Li) and end with francium (Fr).
What are rows and columns called?
Rows are often referred to as Records in Database Management and as Horizontal Arrays in a matrix. Columns are referred to as Fields in Database Management and as Vertical Arrays in a matrix.
Why is it called periodic table?
Why is the periodic table called the periodic table? It is called the periodic table because of the way the elements are arranged. You'll notice they're in rows and columns. The horizontal rows (which go from left to right) are called 'periods' and the vertical columns (going from up to down) are called 'groups'.
How are the columns on the periodic table organized?
A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 3.2. 2). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name.
What is column 18 of the periodic table called?
the noble gasesGroup 18 of the periodic table contains the noble gases. These are the elements in the 18th column of the periodic table, at the far right. They are all nonmetals, and are found in their standard state as monatomic gases. Noble gases are relatively inert and nonreactive due to their full outer shell of electrons.
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
What are the elements that chemists classify?
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
What is the value of an atom in the periodic table?
However, the value given in the periodic table is an average of the mass of all isotopes of a given element. While the number of electrons does not contribute significant mass to an atom, isotopes have differing numbers of neutrons, which do affect mass.
Where are period numbers located in a table?
How to Identify It: Period numbers are located on the left-hand side of the table. These are simple integer numbers. Examples: The row starting with hydrogen is 1. The row starting with lithium is 2.
What is the atomic number of an element?
Element Atomic Number. One number you will find on all periodic tables is the atomic number for each element. This is the number of protons in the element, which defines its identity. How to Identify It: There isn't a standard layout for an element cell, so you need to identify the location of each important number for the specific table.
Why do periodic tables not have periods?
Most periodic tables do not number them because they are fairly obvious, but some tables do. The period indicates the highest energy level att ained by electrons of an atom of the element in the ground state. How to Identify It: Period numbers are located on the left-hand side of the table. These are simple integer numbers.
Why do periodic tables omit electron configuration?
Most tables omit this value because it takes up a lot of room.
What is the lowest atomic number?
The atomic number is easy because it is an integer that increases as you move from left to right across the table. The lowest atomic number is 1 ( hydrogen ), while the highest atomic number is 118. Examples: The atomic number of the first element, hydrogen, is 1. The atomic number of copper is 29.
How to identify atomic mass?
How to Identify It: The atomic mass is a decimal number. The number of significant figures varies from one table to another. It's common to list values to two or four decimal places. Also, the atomic mass is recalculated from time to time, so this value may change slightly for elements on a recent table compared with an older version.