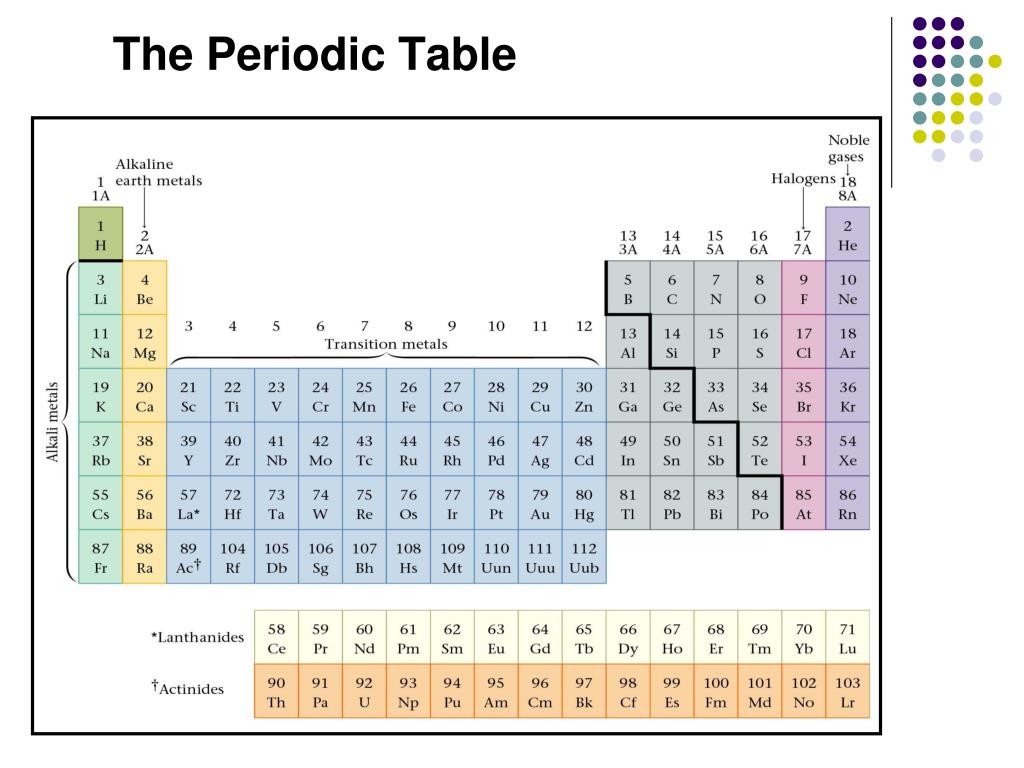
What are the groups and families on the periodic table?
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods. Group (family): A vertical column in the periodic table.
What are examples of families in the periodic table?
oxygen family. halogen family. Regarding this, what are the 7 families of the periodic table? This list includes alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides, as well as seven elements in groups 3 through 6—aluminum, gallium, indium, thallium, tin, lead, and bismuth.
How are families of the periodic table named?
Three systems have been used to number families and groups:
- The older IUPAC system used Roman numerals together with letters to distinguish between the left (A) and right (B) side of the periodic table.
- The CAS system used letters to differentiate main group (A) and transition (B) elements.
- The modern IUPAC system uses Arabic numbers 1-18, simply numbering the columns of the periodic table from left to right.
What is the definition of families on the periodic table?
Element families are indicated by numbers located at the top of the periodic table. An element family is a set of elements sharing common properties. Elements are classified into families because the three main categories of elements (metals, nonmetals, and semimetals) are very broad.
See more
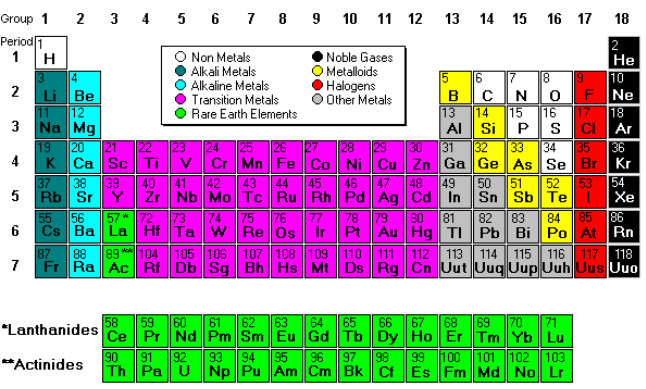
What are element families and element groups?
For the most part, element families and element groups are the same things. Both describe elements that share common properties, usually based on the number of valence electrons. Usually, either family or group refers to one or more columns of the periodic table.
How many valence electrons does a halogen have?
The halogens have 7 valence electrons, while the noble gasses have 8 valence electrons (or 0, depending on how you look at it).
What is an element family?
Element families are elements that have the same number of valence electrons. Most element families are a single column of the periodic table, although the transition elements consist of several columns, plus the elements located below the main body of the table. An example of an element family is the nitrogen group or pnictogens.
Do element groups always have the same number of valence electrons?
Element groups, defined this way, do not always have the same number of valence electrons. For example, the halogens and noble gasses are distinct element groups, yet they also belong to the larger group of nonmetals.
Which group of elements have valence electrons in more than one shell?
They have valence electrons in more than one shell and exhibit several common oxidation states. Groups 13, 14, & 15 include the other metals elements. They are malleable and ductile but are not the same as transition elements.
What are the elements in Group 2?
Group 2 includes the alkaline earth elements. They are metallic elements and have an oxidation number of +2, making them very reactive. Groups 3 through 12 include 38 elements called transition metals. They are malleable and ductile and also conduct heat and electricity.
How many electrons are in Group 17?
Group 17 includes the halogens and are five non-metallic elements. They have 7 electrons in their outer shells and an oxidation number of -1.
How many families are there in the periodic table?
The Periodic table can be divided into nine families of elements each having similar properties. The families include: Alkali Metals. Group 1 of the periodic table are the alkali metals. They are highly reactive and do not occur freely in nature.
What are metalloids?
Metalloids are found between the metals and non-metals along a boundary. They have properties of both metals and non-metals. Some of the metalloids, such as silicon and germanium, are semi-conductors.
Where are lanthanide and actinide found?
These can be found in group 3 of the periodic table, and the 6th and 7th periods.
Do metals have valence electrons?
They do not exhibit a variety of oxidation states, they have valence electrons only in the outer shells. They are solid, have high density, and are opaque. Their oxidation numbers are +3, +/14, and -3. Metalloids are found between the metals and non-metals along a boundary.
What Is a Family on the Periodic Table?
Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels.
How to identify an element family?
Probably the best way to identify an element family is to associate it with an IUPAC group, but you'll find references to other element families in the literature. At the most basic level, sometimes the families are simply considered the metals, metalloids or semimetals, and nonmetals. Metals tend to have positive oxidation states, high melting and boiling points, high density, high hardness, high density, and be good electrical and thermal conductors. Nonmetals, on the other hand, tend to be lighter, softer, have lower melting and boiling points, and be poor conductors of heat and electricity. In the modern world, this is problematic because whether an element has metallic character or not depends on its conditions. For example, hydrogen can act as an alkali metal rather than a nonmetal. Carbon can act as a metal rather than a nonmetal.
Why are homologs used in chemistry?
Element homologs are members of the same element family. Because homologous elements share similar electrochemical properties, they can be used to predict the behavior of new elements. This becomes increasing helpful for the superheavy elements, of which only a few atoms have been prepared. However, predictions are not always accurate. The reason is because valence electron effects aren't quite as significant when an atom has extremely high numbers of both protons and electrons. Lighter homologs more often share common properties.
How many electrons are in the valence shell of an element?
These elements have 8 electrons in the valence shell (a complete octet). Group 1 is also known as the alkali metals or the lithium group. Elements in this group have one orbital electron in the outer shell. Group 16 is also known as the oxygen group or chalcogen family.
Why are element groups labelled by number?
Family members share similar chemical and physical properties. An element family is also called an element group. Because of the potential for confusion, the IUPAC prefers element groups be labelled by number rather than name.
What is the primary factor in predicting the types of reactions an element will participate in?
This is because the number of valence electrons is the primary factor in predicting the types of reactions an element will participate in, the bonds it will form, its oxidation state, and many of its chemical and physical properties. Examples: Group 18 on the periodic table is also known as the noble gas family or noble gas group.
Why are nonmetals considered to be poor conductors of heat and electricity?
In the modern world, this is problematic because whether an element has metallic character or not depends on its conditions .
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
Why is period 1 the shortest period?
Period 1 of the periodic table is given the name shortest period because there are only two elements in period 1.
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).