
Which way does an a period go in the periodic table?
Summary The periodic table is arranged in order of atomic number A period is a horizontal row of the periodic table. A group is a vertical row of the periodic table.
What does each period in the periodic table correspond to?
What does each period in the periodic table corresponds to? Each period corresponds to a principal energy level. it is arranged in order of increasing atomic mass. All of the elements in a period have the same number of atomic orbitals. For example, every element in the top row (the first period) has one orbital for its electrons.
How much periods does a periodic table have?
The Periodic Table consists of seven periods, from Period 1 to Period 7. Table shows the changes in the proton numbers and number of valence electrons when going across Period 2. the proton number increases by one from one element to the next
What does group and period represent in the periodic table?
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period. Thereof, what does group number in periodic table mean?
See more
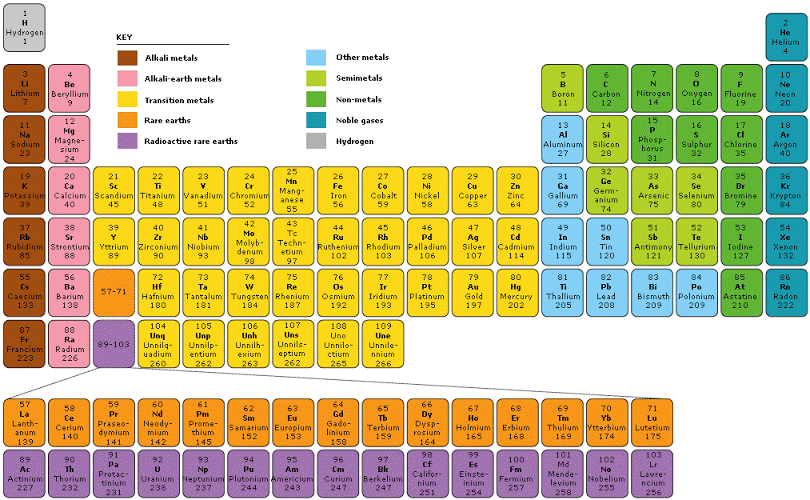
What is a period on the periodic?
The horizontal rows of the periodic table are called periods. Each period corresponds to the successive occupation of the orbitals in a valence shell of the atom, with the long periods corresponding to the occupation of the orbitals of a d subshell.
What is period and group in periodic table?
The vertical columns in the periodic table are called 'groups' and horizontal rows in the table are called periods.
What is period in periodic table example?
A period is a horizontal row of elements on the periodic table. For example, the elements sodium (Na) and magnesium (Mg) are both in period 3. The elements astatine (At) and radon (Rn) are both in period 6.
What is difference between group and period?
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
How do you remember periods and groups?
0:292:53Periods & Groups In The Periodic Table | Properties of Matter - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach new period row represents a new shell elements in the first period have one shell and as we goMoreEach new period row represents a new shell elements in the first period have one shell and as we go down the shells.
How many periods are there?
Between the ages of 12 and 52, a woman will have around 480 periods, or fewer if she has any pregnancies.
How many periods are on the periodic table?
seven periodsThere are seven periods in the periodic table, with each one beginning at the far left. A new period begins when a new principal energy level begins filling with electrons. Period 1 has only two elements (hydrogen and helium), while periods 2 and 3 have 8 elements.
How many groups and periods are on the periodic table?
There are eighteen vertical columns known as groups in the modern periodic table which are arranged from left to right and seven horizontal rows which are known as periods.