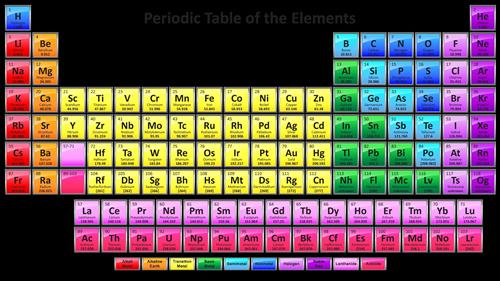
What does period mean in a periodic table?
The horizontal rows of the periodic table are called periods. Each period corresponds to the successive occupation of the orbitals in a valence shell of the atom, with the long periods corresponding to the occupation of the orbitals of a d subshell.
What is period in periodic table example?
A period is a horizontal row of elements on the periodic table. For example, the elements sodium (Na) and magnesium (Mg) are both in period 3. The elements astatine (At) and radon (Rn) are both in period 6.
What is group and period?
The vertical columns in the periodic table are called 'groups' and horizontal rows in the table are called periods.
How do you remember periods and groups?
0:292:53Periods & Groups In The Periodic Table | Properties of Matter - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach new period row represents a new shell elements in the first period have one shell and as we goMoreEach new period row represents a new shell elements in the first period have one shell and as we go down the shells.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
Why is period 1 the shortest period?
Period 1 of the periodic table is given the name shortest period because there are only two elements in period 1.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
What is the periodic table?
There is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. Generally, within one row (period) the elements are metals to the left, and non-metals to the right, with the elements having similar chemical behaviours placed in the same column.
What is the total number of protons in an atom called?
The total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol Z. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19coulombs. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What are the rows of lanthanoid and actinoid?
These rows contain elements in the lanthanoid and actinoid series, usually from 57 to 71 ( lanthanum to lutetium) and 89 to 103 ( actinium to lawrencium ), respectively. There is no scientific reason for this. It is merely done to make the table more compact.
What is the atomic number of an element?
The atomic number of an element is the number of protons in the nucleus of an atom of that element . Hydrogen has 1 proton, and oganesson has ...
What is the periodic table?
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, ...
What elements are triads?
Döbereiner in 1817 showed that the combining weight, meaning atomic weight, of strontium lies midway between those of calcium and barium, and some years later he showed that other such “ triads ” exist (chlorine, bromine, and iodine [halogens] and lithium, sodium, and potassium [alkali metals]). J.-B.-A. Dumas, L. Gmelin, E. Lenssen, Max von Pettenkofer, and J.P. Cooke expanded Döbereiner’s suggestions between 1827 and 1858 by showing that similar relationships extended further than the triads of elements, fluorine being added to the halogens and magnesium to the alkaline-earth metals, while oxygen, sulfur, selenium, and tellurium were classed as one family and nitrogen, phosphorus, arsenic, antimony, and bismuth as another family of elements.
Why do the elements in the periodic table have different orbits?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion principle, no more than two electrons can fill the same orbital. The first row of the periodic table consists of just two elements, hydrogen and helium. As atoms have more electrons, they have more orbits available to fill, and thus the rows contain more elements farther down in the table.
How many protons does hydrogen have?
The atomic number of an element is the number of protons in the nucleus of an atom of that element. Hydrogen has 1 proton, and oganesson has 118.
What are the elements that are related to the first seven?
Newlands proposed classifying the elements in the order of increasing atomic weights, the elements being assigned ordinal numbers from unity upward and divided into seven groups having properties closely related to the first seven of the elements then known: hydrogen, lithium, beryllium, boron, carbon, nitrogen, and oxygen . This relationship was termed the law of octaves, by analogy with the seven intervals of the musical scale.
What are the different types of nonmetals?
The nonmetals, halogens, and noble gases are all types of nonmetals. The metalloids have properties intermediate between metals and nonmetals. The alkali metals, alkaline earths, lanthanides, actinides, transition metals, and basic metals are all groups of metals.
What are the elements that chemists classify?
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
How are atoms determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
What is the atomic radius of oxygen?
The atomic radius of Oxygen atom is 66pm (covalent radius).
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.