Why is the periodic table so important?
Why Learn Periodic Table?
- It predicts the properties of different elements
- The Columns called as groups and rows are known as periods indicate the elemental characteristics
- The table makes different trends with the properties of elements
- The table gives a piece of information as to how the chemical equations are balanced.
What are some interesting facts about the periodic table?
Interesting Facts On Periodic Table of Elements
- Founder of Periodic Table. Dmitri Mendeleyev is the father of the modern periodic table of elements. ...
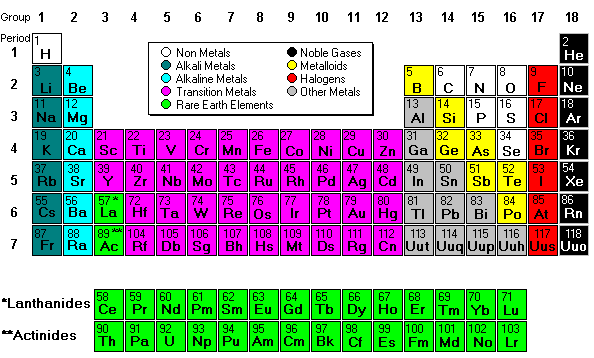
- Columns of the Periodic Table. The periodic table has 18 vertical columns called groups and seven horizontal columns called Periods.
- Size of the Atom. ...
- Unique Elements. ...
- Properties of Elements. ...
- Facts About Hydrogen. ...
What are the first 20 elements on the periodic table?
What are the first 20 elements in order?
- H – Hydrogen
- He – Helium
- Li – Lithium
- Be – Beryllium
- B – Boron
- C – Carbon
- N – Nitrogen
- O – Oxygen
- F – Fluorine
- Ne – Neon
What are the basic elements of the periodic table?
- Element 13 - Aluminum
- Element 31 - Gallium
- Element 49 - Indium
- Element 50 - Tin
- Element 81 - Thallium
- Element 82 - Lead
- Element 83 - Bismuth
- Element 113 - Ununtrium - will probably be a basic metal.
- Element 114 - Flerovium - will probably be a basic metal.
- Element 115 - Ununpentium - will probably be a basic metal.

What is the periodic table simple definition?
What is the periodic table? The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson.
What is the periodic table definition for kids?
The periodic table is a system for arranging the chemical elements. The chemical elements are the basic substances that make up all matter. Each chemical element has a particular feature called its atomic number. That number comes from the amount of tiny particles called protons in each atom of the element.
What is the periodic table table?
The periodic table of chemical elements, often called the periodic table, organizes all discovered chemical elements in rows (called periods) and columns (called groups) according to increasing atomic number.
Why is it called the periodic table?
Why is the periodic table called the periodic table? It is called the periodic table because of the way the elements are arranged. You'll notice they're in rows and columns. The horizontal rows (which go from left to right) are called 'periods' and the vertical columns (going from up to down) are called 'groups'.
What are 5 facts about the periodic table?
15 Fun and Surprising Facts About the Periodic Table of ElementsDmitri Mendeleyev is the inventor of the modern periodic table. ... Scientists used battery polarity to weigh the elements. ... The periodic table reflects its creator's love for card games. ... It was used to correctly predict elements that hadn't been discovered.More items...•
How do I teach my 5 year old the periodic table?
5 Fun Ways to Teach the Periodic TableTry a merka Learning Kit. ... Go Over It Each Day (in Song) ... Play an Educational App. ... Use Fun Fact Flash Cards. ... Build Your Own with Post-It Notes.
What is periodic table used for?
It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit a periodic dependence on their atomic numbers.
Why is the periodic table important?
The periodic table of elements puts all the known elements into groups with similar properties. This makes it an important tool for chemists, nanotechnologists and other scientists. If you get to understand the periodic table, and learn to use it, you'll be able to predict how chemicals will behave.
What are the main features of the periodic table?
The Periodic Table organizes the elements according to their similar chemical and physical properties. The Table has rows and columns. The vertical columns in the periodic table represent Groups. The horizontal rows in table represents Periods.
Who invented periodic table?
Dmitri MendeleevAlbert GhiorsoPeriodic table/Inventors
What is rarest element on earth?
element astatineA team of researchers using the ISOLDE nuclear-physics facility at CERN has measured for the first time the so-called electron affinity of the chemical element astatine, the rarest naturally occurring element on Earth.
How the periodic table is arranged?
The periodic table is arranged by atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row (called a period) and column (called a group). The table comprises seven rows and 18 columns.
What is the periodic table?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen...
What do periodic table groups have in common?
The groups of the periodic table are displayed as vertical columns numbered from 1 to 18. The elements in a group have very similar chemical proper...
Where does the periodic table come from?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion princ...
Why does the periodic table split?
The periodic table has two rows at the bottom that are usually split out from the main body of the table. These rows contain elements in the lantha...
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
How many categories are there in the periodic table?
The elements of the periodic table shown here are divided into nine categories; six for the metals, and two for nonmetals, and a metalloid category. The nine categories (or sets) correspond to those found in the literature for the applicable part of the periodic table. Different authors may use different categorisation schema depending on the properties of interest.
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What is the electron configuration of a neutral atom?
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered 1, 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram. The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
When is the 150th anniversary of the periodic table?
In celebration of the periodic table's 150th anniversary, the United Nations declared the year 2019 as the International Year of the Periodic Table, celebrating "one of the most significant achievements in science".
What is the periodic table?
There is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. Generally, within one row (period) the elements are metals to the left, and non-metals to the right, with the elements having similar chemical behaviours placed in the same column.
What is the total number of protons in an atom called?
The total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol Z. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19coulombs. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
What is PubChem working with?
PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the Periodic Table?
The periodic table is an arrangement of all the elements known to man in accordance with their increasing atomic number and recurring chemical properties. They are assorted in a tabular arrangement wherein a row is a period and a column is a group.
How many elements are naturally occurring in the periodic table?
Therefore, as the energy level of the atom increases, the number of energy sub-levels per energy level increases. The first 94 elements of the periodic table are naturally occurring, while the rest from 95 to 118 have only been synthesized in laboratories or nuclear reactors.
What is the difference between the modern periodic law and the Mendeleev periodic table?
Mendeleev modeled his periodic table on the basis of increasing atomic mass, whereas, the modern periodic law is based on the increasing order of atomic numbers. Even though Mendeleev’s periodic table was based on atomic weight, he was able to predict the discovery and properties of certain elements. During his time only around half of the elements ...
How are elements arranged in order?
Elements are arranged from left to right and top to bottom in the order of their increasing atomic numbers. Thus,
When was Mendeleev's periodic table published?
Mendeleev’s Periodic Table was published in the German Journal of chemistry in 1869 .
When was iodine discovered?
Iodine was discovered by Bernard Courtois in 1811.
What is the periodic table?
The periodic table is a tabular arrangement of the chemical elements by increasing atomic number which displays the elements so that one may see trends in their properties.
How are periodic tables arranged?
Key Takeaways: Periodic Table Definition 1 The periodic table is a tabular arrangement of chemical elements that is arranged by increasing atomic number and groups elements according to recurring properties. 2 The seven rows of the periodic table are called periods. The rows are arranged so that metals are on the left side of the table and nonmetals are on the right side. 3 The columns are called groups. Group contain elements with similar properties.
What are the elements that make up the majority of the periodic table?
The majority of chemical elements are metals. Metals tend to be shiny (metallic luster), hard, conductive, and capable of forming alloys. Nonmetals tend to be soft, colored, insulators, and capable of forming compounds with metals. Metalloids display properties intermediate between those of metals and nonmetals. Toward the right side of the periodic table, the metals transition into nonmetals. There is a rough staircase pattern—starting at boron and going through silicon, germanium, arsenic, antimony, tellurium, and polonium—that identified the metalloids. However, chemists increasingly categorize other elements as metalloids, including carbon, phosphorus, gallium, and others.
What are the rows on the periodic table called?
The seven rows of the periodic table are called periods . The rows are arranged so that metals are on the left side of the table and nonmetals are on the right side. The columns are called groups. Group contain elements with similar properties.
How many rows are there in the periodic table?
There are seven rows of the periodic table, which are called periods. Element atomic number increases moving from left to right across a period. Elements toward the left side of a period are metals, while those on the right side are nonmetals. Moving down a period on the table adds a new electron shell.
Where are the elements on the periodic table?
Metallic character is highest at the bottom lefthand corner of the periodic table, while the most nonmetallic elements are in the upper righthand corner.
What are the columns of elements called?
The columns of elements are called groups or families . Groups are numbered from 1 (the alkali metals) to 18 (the noble gases). Elements with a group share a valence electron configuration. Elements within a group display a pattern with respect atomic radius, electronegativity, and ionization energy.
How many elements are in the periodic table?
Since then, the periodic table has evolved to reflect over 150 years of scientific development and understanding in chemistry and physics. Today, with 118 known elements, it is widely regarded as one of the most significant achievements in science.
What is the name of the table of chemical elements?
Periodic Table of Chemical Elements. The periodic table of chemical elements, often called the periodic table, organizes all discovered chemical elements in rows (called periods) and columns (called groups) according to increasing atomic number.
Why do scientists use the periodic table?
Scientists use the periodic table to quickly refer to information about an element, like atomic mass and chemical symbol. The periodic table’s arrangement also allows scientists to discern trends in element properties, including electronegativity, ionization energy, and atomic radius.
What is the periodic table?
periodic table. The periodic table is a system for arranging the chemical elements. The chemical elements are the basic substances that make up all matter. Each chemical element has a particular feature called its atomic number.
How does the periodic table work?
The periodic table arranges the elements in rows and columns. In the rows, the elements are placed in order of their atomic number . The columns form groups of elements that have similar chemical properties. For example, certain gases are in one column and metals are in another. The periodic table helps chemists think about ...
Why is the periodic table important?
For example, certain gases are in one column and metals are in another. The periodic table helps chemists think about the elements and their properties. A Russian chemist named Dmitry Mendeleyev developed the first periodic table in 1869. At the time, scientists did not know about atomic numbers.
Did scientists know about atomic numbers?
At the time, scientists did not know about atomic numbers. They did know that each element had an atomic weight, however. Mendeleyev noticed that there is a relationship between the atomic weights and other properties of the elements.
How many elements are there in the periodic table?
Of course, the periodic table is all about the elements. The elements are identified by the number of protons in an atom of that element. Right now, you'll see 118 elements on the periodic table, but as more elements are discovered, another row will be added to the table.
Why is the periodic table important?
The periodic table of the elements is one of the most important tools used by chemists and other scientists because it summarizes useful information about the chemical elements in a format that shows relationships between the elements.
Why are periodic tables good?
Printed periodic tables are great because you can mark them up and not worry about ruining your book.
Who is the father of the periodic table?
Periodic Table History. Many people consider Dmitri Mendeleev to be the Father of the Modern Periodic Table. Mendeleev's table was slightly different from the table we use today in that his table was ordered by increasing atomic weight and our modern table is ordered by increasing atomic number.
Can you be tested on the periodic table?
Because it's necessary to know what the periodic table is and how to use it, you can expect to be tested about it from grade school pretty much until the end of time. Before your grade is on the line, probe your strengths and weaknesses with online quizzes. You might even have fun!

Summary
The periodic table, also known as the periodic table of the (chemical) elements, is a tabular display of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit a periodic dependence on their atomic numbers. Th…
Overview
The periodic table is a 2-dimensional structured table. The elements are placed in table cells, in reading order of ascending atomic number. The table columns are called groups, the rows are called periods. The breaks at the end of each period occur according to a repetition (or periodicity) of physical and chemical properties of the elements.
Periodic trends
As chemical reactions involve the valence electrons, elements with similar outer electron configurations may be expected to react similarly and form compounds with similar proportions of elements in them. Such elements are placed in the same group, and thus there tend to be clear similarities and trends in chemical behaviour as one proceeds down a group. As analogous configurations return …
Classification of elements
Many terms have been used in the literature to describe sets of elements that behave similarly. The group names alkali metal, alkaline earth metal, pnictogen, chalcogen, halogen, and noble gas are acknowledged by IUPAC; the other groups can be referred to by their number, or by their first element (e.g., group 6 is the chromium group). Some divide the p-block elements from groups 13 to …
History
In 1817, German physicist Johann Wolfgang Döbereiner began to formulate one of the earliest attempts to classify the elements. In 1829, he found that he could form some of the elements into groups of three, with the members of each group having related properties. He termed these groups triads. Chlorine, bromine, and iodine formed a triad; as did calcium, strontium, and barium; lithi…
Current questions
Although the modern periodic table is standard today, some variation can be found in period 1 and group 3. Discussion is ongoing about the placements of the relevant elements. The controversy has to do with conflicting understandings of whether chemical or electronic properties should primarily decide periodic table placement, and conflicting views of how the evidence should be used. A similar potential problem has been raised by theoretical investigations of the superheav…
Future extension beyond the seventh period
The most recently named elements – nihonium (113), moscovium (115), tennessine (117), and oganesson (118) – completed the seventh row of the periodic table. Future elements would have to begin an eighth row. These elements may be referred to either by their atomic numbers (e.g. "element 119"), or by the IUPAC systematic element names which directly relate to the atomic …
Alternative periodic tables
The periodic law may be represented in multiple ways, of which the standard periodic table is only one. Within 100 years of the appearance of Mendeleev's table in 1869, Edward G. Mazurs had collected an estimated 700 different published versions of the periodic table. Many forms retain the rectangular structure, including Janet's left-step periodic table (pictured below), and the m…