What does each row on the periodic table tell us?
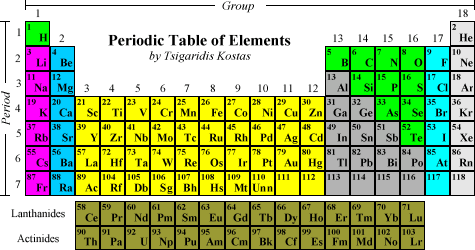
An element's position on the table indicates which elements share its basic properties. The Periodic Table is organized according to atomic number, which is equal to the number of protons found in the nuclei of an element's atoms. The rows on the table are known as periods, and the columns are known as groups. Elements in certain groups tend to share characteristics.
What do the rows mean on the periodic table mean?
The rows on the table are known as periods and the elements in each row also have similarities. The similarity is the number of atomic orbitals that each element contains. The elements in row 1 contain 1 orbital that the electrons appear in, row 2 has 2 orbitals and this carries on down the rows.
What does each row of the periodic table end with?
each row in the periodic table ends with a... noble gas. in going from left to right in any given row in the periodic table, the size of atoms generally... decreases. compared to the neutral atom from which it is derived, a negative ion is... always larger.
What are horizontal rows in the periodic table known as?
The horizontal rows in the periodic table are periods, while the vertical rows are called groups. What are the rows on the periodic table of elements also known as? The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups, contain elements with similar chemical behaviours.

Why is a row called a period?
When you look at the periodic table, each row is called a period (Get it? Like PERIODic table.). All of the elements in a period have the same number of atomic orbitals. For example, every element in the top row (the first period) has one orbital for its electrons.
What are columns called on the periodic table?
The rows of the table are called periods, and the columns are called groups. Elements from the same column group of the periodic table show similar chemical characteristics.
What are the 7 rows in the periodic table called?
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups.
What are rows and columns called?
Columns or rows in databases A combination of database columns and rows is knowns as a table. Each database table row has the same set of data fields.
What is the first column in the periodic table called?
Alkali metals: The alkali metals make up most of Group 1, the table's first column. Shiny and soft enough to cut with a knife, these metals start with lithium (Li) and end with francium (Fr).
What is column 18 of the periodic table called?
Groups are numbered 1–18 from left to right. The elements in group 1 are known as the alkali metals; those in group 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in 18 are the noble gases.
What is the F block called?
the inner transition elementsThe f block elements are the lanthanides and actinides and are called the inner transition elements because of their placement in the periodic table due to their electron configurations.
What are the 7 groups of the periodic table?
Different groups are present in the periodic table:The Alkali Metals.The Alkaline Earth Metals.The Transition Metals.The Non-metals.The Halogens.The Noble Gases.The Rare Earth Elements.