
Full Answer
How many elements are in transition metals?
There are total of 38 elements in this group including Cobalt, Nickel, Iron, Rhodium, Gold, Silver, Cooper, Scandium, Titanium, Vanadium, Manganese, Zinc and Mercury. https://en.wikipedia.org/wiki/Transition_metal
What elements are transition metals?
There are a number of elements that are classified as transition metals. They occupy columns 3 through 12 of the periodic table and include such metals as titanium, copper, nickel, silver, platinum, and gold . Sometimes included in the transition metal group are the lanthanides and actinides. They are called the "inner transition metals."
What is a transitional metal on the periodic table?
The IUPAC definition defines a transition metal as "an element whose atom has a partially filled d sub-shell, or which can give rise to cations with an incomplete d sub-shell". Many scientists describe a "transition metal" as any element in the d-block of the periodic table, which includes groups 3 to 12 on the periodic table.
What are transition metals list?
Transition Metal List. A list of transition metals is found below divided by each group: Group 3: scandium (Sc), yttrium (Y), lutetium (Lu), lawrencium (Lr)
What is a transition metal simple definition?
Definition of transition metal : any of various metallic elements (such as chromium, iron, and nickel) that have valence electrons in two shells instead of only one.
What are the transition metals located on the periodic table?
The transition elements or transition metals occupy the short columns in the center of the periodic table, between Group 2A and Group 3A.
What is a transition metal example?
Examples of Transition Metals Some of the most common examples include iron, chromium, manganese, vanadium, titanium, copper, cobalt, nickel, tungsten, gold, and platinum.
Why are they called transition metals?
Transition metals are found in the periodic table between the s-block and p-block elements. Thus, they are called d-block elements. Transition metals are unstable metals that display transitional behavior between s and p block elements, thus their name.
What are transition metals known for?
Some of the main shared properties of transition metals can be identified as the below:They form coloured compounds.They are good conductors of heat and electricity.They can be bent into shape easily.They are less reactive than alkali metals.They have high melting points.More items...•
How do you identify transition elements?
Early transition metals are on the left side of the periodic table from group 3 to group 7. Late transition metals are on the right side of the d-block, from group 8 to 11 (and 12 if it is counted as transition metals).
Is Group 3 a transition metal?
Group 3 is the first group of transition metals in the periodic table.
What and where are transition metals?
The main group elements include the active metals in the two columns on the extreme left of the periodic table and the metals, semimetals, and nonmetals in the six columns on the far right. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table.
What is another name for the transition metals?
group B elementsAnswer and Explanation: The transition metals are also known as the group B elements. The transition metals occupy the middle section of the periodic table. They contain the metallic elements such as iron, silver, and gold.
How many transition metals are there?
38 elementsThere are total of 38 elements in this group including Cobalt, Nickel, Iron, Rhodium, Gold, Silver, Cooper, Scandium, Titanium, Vanadium, Manganese, Zinc and Mercury. Transition metals are characterized by properties not found in other groups on the periodic table.
Why is iron a transition metal?
These elements lie in the middle of periodic table between s and p-blocks (i.e., between group 2 and group 13). A transition element may be defined as a element whose atom or at least one of its simple ions contain partially filled d-orbitals, e.g., iron, copper, chromium, nickel etc.
How many transition series of elements are there in the periodic table?
Hence there are a total thirty-eight (38)transition elements in the periodic table.
Where are transition metals found?
Transition metals are found everywhere on Earth in various amounts. Most are not found in a pure substance, but rather in compounds buried in the Earth's crust.
How many transition metals are there?
38 elementsThere are total of 38 elements in this group including Cobalt, Nickel, Iron, Rhodium, Gold, Silver, Cooper, Scandium, Titanium, Vanadium, Manganese, Zinc and Mercury. Transition metals are characterized by properties not found in other groups on the periodic table.
Why are transition metals in the middle of the periodic table?
The transition metals are located in the middle of the periodic table in between the elements of the left and the right. They act as a bridge or transition between the two sides of the periodic table.
What are transition metals give four examples?
orbitals are known as transition elements. All of them are metals.
eg : chromium, manganese, vanadium and copper.
What is transition metal?
One definition of a transition metal, is any metal that has at least one unpaired d electron in one of their stable ions. Unpaired d electrons are more likely to participate in chemical reactions. This definition excludes scandium, since the Sc+3 ion does not have unpaired d electrons.
Why are transition metals colored?
Transition metal compounds are often highly colored, due to d to d electron transitions. They often form paramagnetic compounds because of their unpaired d electrons. In their elemental form, they often act as catalysts.
What format did the original periodic table use?
The original periodic table featured periodicity in a simple 8-column format. In other words unlike the current periodic table that recognizes the increasing length of periods as atomic number increases, (2, 8, 18, 32 etc.) the original table attempted to squeeze all the elements into an 8-column format. In order to do this Mendeleev and other pioneers of the periodic table were obliged to remove certain elements from the main body of the table and had to create a special group VIII which featured “transition elements” including Fe, Co, Ni, Ru, Rh, Pd, Os, In, Pt.
Who first used the term "elements"?
The English chemist Charles Bury first used this term, to describe this group of elements.
How many oxidation states does manganese have?
Almost all exhibit multiple oxidation states, especially the metals in groups 5,6,7, and 8. For example, manganese can easily be put into 5 different oxidation states.
Where are transition metals located in the periodic table?
June 10, 2021 September 14, 2020 by Admin. The transition metals are located in the middle of the Periodic table from group 3 to group 11. From the above image, you can easily see where are Transition Metals located on the Periodic Table. According to some chemists, the d-block elements are known as transition elements.
How many transition metals are there in the periodic table?
There are 31 commonly known transition metals on the periodic table as shown in the above image by yellow color. (According to the definition given by IUPAC)
What is the transition from metallic to nonmetallic?
In other words, the transition of metallic nature to nonmetallic nature appears in these elements. As the properties of these elements show transition from electropositive nature to electronegative nature, they are called transition metals. Definition by IUPAC. Transition elements (or transition metals) are those elements which have partially ...
Why are transition elements called transition elements?
Here is a reason: Transition elements are called so because their properties show the transition from electropositive s-block elements to the electronegative p-block elements. These elements form a bridge between the best metals and the best nonmetals. (Metals are on the left side of periodic table and nonmetals are on the right side ...
Why are group 12 elements not considered transition metals?
According to the definition given by IUPAC, the group 12 elements (Zn, Cd and Hg) are not considered as transition metals because they do not have incomplete d-orbitals either in their elemental state (Zn, Cd, Hg) or oxidation state (Zn 2+, Cd 2+, Hg 2+ ). Read: The exact reason why group 12 elements are not included as transition elements? (Explained with examples)
Where are transition metals located?
Well, transition metals are located in the middle of the Periodic table from group 3 to group 11.
Which group of elements are transition metals?
Finally as a summary, simply remember that the elements lying from group 3 to 11 are the transition metals on the periodic table.
Why are transition metals called transition metals?
These elements are called " transition metals " because the electrons of their atoms make the transition to filling the d subshell or d sublevel orbital. Thus, the transition metals are also known as the d-block elements. Here is a list of elements that are considered to be transition metals or transition elements.
Which transition metals have high melting points?
The transition metals, as a group, have high melting points. The exception is mercury, which is a liquid at room temperature. By extension, these elements also have high boiling points. Their d orbitals become progressively filled as you move from left to right across the periodic table.
Why do transition metals have colored complexes?
Transition metals form colored complexes, so their compounds and solutions may be colorful. The complexes split the d orbital into two energy sublevels so that they absorb specific wavelengths of light. Because of the different oxidation states, it's possible for one element to produce complexes and solutions in a wide range of colors.
What is the largest group of elements on the periodic table?
The largest group of elements on the periodic table is that of the transition metals, which is found in the middle of the table. Also, the two rows of elements below the main body of the periodic table (the lanthanides and actinides) are special subsets of these metals. These elements are called " transition metals " because the electrons ...
What is the oxidation state of iron?
For example, iron commonly carries a 3+ or 2+ oxidation state. Copper may have a 1+ or 2+ oxidation state. The positive oxidation state means the transition metals typically form ionic or partially ionic compounds. Atoms of these elements have low ionization energies.
Is transition metal a conductor of heat?
They are excellent conductors of heat and electricity. The transition metals are malleable (easily hammered into shape or bent). These metals tend to be very hard. Transition metals look shiny and metallic.
Is a transition metal reactive?
Although the transition metals are reactive, they are not as reactive as elements belonging to the alkali metals group. Many transition metals form paramagnetic compounds. Helmenstine, Anne Marie, Ph.D. "Transition Metals: List and Properties.".
What are transition metals?
The transition metals are in the block in the middle of the Periodic Table, between Groups 2 and 3. They are all metals and include many common metals such as chromium (Cr), iron (Fe), nickel (Ni) and copper (Cu). Transition metals form ions with different charges.
Why is the periodic table important?
The Periodic Table helps to categorise the known elements and make predictions about ones that we haven’t yet discovered.
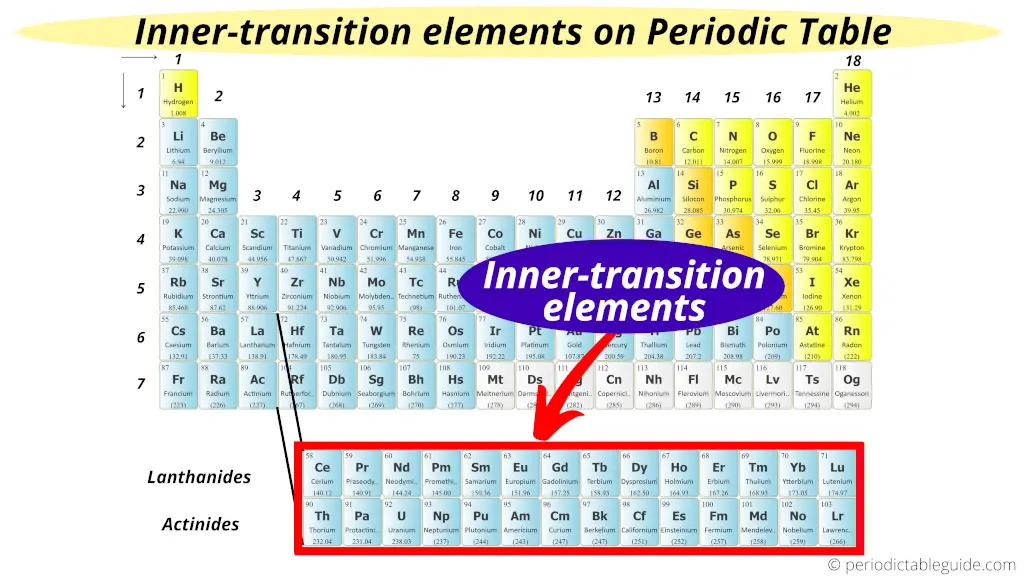
Why Inner Transition Elements are called so?
Because they have similar properties like transition elements, and they are placed in the inner section as an extension of group 3.
Where are inner transition metals located?
Inner transition metals are located in the two rows at the bottom of the periodic table.
What happens if the inner transition elements are not placed in the bottom?
If the inner transition elements were not placed in the bottom, then there will be a longer periodic table like this.
What metals are used to make nuclear weapons?
Uranium and plutonium are inner transition metals which are used for manufacturing nuclear weapons.
Which elements are placed separately in the two rows at the bottom of the periodic table?
They have their valence electrons in the f-orbitals. Hence, the transition metals (lanthanoids and actinoids) are placed separately in the two rows at the bottom of the Periodic table. And as inner transition elements have valence electrons in f-orbitals, ...
What metals are used to make magnets?
Neodymium (Nd), Cerium (Cm) and Samarium (Sm) are mixed with other metals to prepare strong magnets.
What is the atomic number of lanthanides?
Lanthanides (Ce – Lu, having atomic number from 58 – 71) and

What Are The Transition Metals?
Properties of The Transition Metals
- Transition metals are generally good conductors of heat and electricity, malleable and ductile. Their compounds are often brightly colored in solution and when hydrated, and can exhibit multiple positive oxidation states. They are hard solids, with high melting points and boiling points. Their compounds are often paramagnetic. They are usually quite dense, and are less rea…
Examples of Transition Metals
- Some of the most common examples include iron, chromium, manganese, vanadium, titanium, copper, cobalt, nickel, tungsten, gold, and platinum.
Transition Metal Chemistry
- Transition metal compounds are often highly colored, due to d to d electron transitions. They often form paramagnetic compounds because of their unpaired d electrons. In their elemental form, they often act as catalysts. Almost all exhibit multiple oxidation states, especially the metals in groups 5,6,7, and 8. For example, manganese can easily be put into 5 different oxidation state…
Why Are They So Colorful?
- It is because of their unfilled d orbitals, and something called “d to d electronic transitions”. When a transition metal forms an ion, its electrons can absorb light and move between d orbitals. The d orbitals are normally degenerate, meaning they are all at the same energy level. But when a transition metal forms a complex with a ligand, such as H2O or NH3, the d orbitals develop diffe…
Fun Facts About The Transition Metals
- Iridium can form compounds in the +9 oxidation state is rare circumstances
- Chromium and manganese form powerful oxidizing agents, the dichromate and permanganate ions
- Osmium tetroxide is a volatile solid that melts at 40C and is highly toxic
- Rhodium is the most expensive metal and costs over $800 a gram in 2021
Why Transition Elements Are called so?
How Many Transition Metals Are There in The Periodic table?
Electronic Configuration of Transition Metals
Transition Metals Facts
- The facts of transition elements are mentioned below. 1. Transition elements can have valence electronsin the shell other than the outer shell. 2. Transition metals like iron (Fe), zinc (Zn) and copper (Cu) provide necessary nutrients for human health. 3. Iron (Fe), Cobalt (Co) and nickel (Ni) are those transition metals which produce magnetic fiel...
Transition Metals Uses
Free Gift For You: Interactive Periodic Table
Summary