What is 14 on the periodic table?
The carbon family is element group 14 of the periodic table. The carbon family consists of five elements: carbon, silicon, germanium, tin, and lead. It is likely that element 114, flerovium, will also behave in some respects as a member of the family.
What are the cations and anions in the periodic table?
Main Differences Between Cation and Anion
- Cation is generally a positively charged atom, whereas Anion is a negatively charged atom.
- Cation is generally smaller in size than the Anion, whereas Anion is generally larger.
- Cation has fewer electrons than the protons, whereas Anion has higher numbers of electrons than the protons.
How many elements can you find on a periodic table?
Unless you currently work in a field that involves science, the days of memorizing the elements of the period table are long behind you. There are currently 118 known elements on the periodic table, and we’ve put 20 of them below to test your smarts.
What is the 18 element in the periodic table?
Today, I will present the first eighteen elements which are Hydrogen,Helium, Lithium,Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Sodium, Magnesium, Aluminum, Silicon, Phosphorus, Sulfur, Chlorine, and Argon. Furthermore, what is O in periodic table?
What is a ion and example?
An ion is an atom or group of atoms where the number of electrons is not equal to the number of protons. That means when a stable atom gains or loses an electron, it becomes an ion. Examples of ions are as follows: H+,Na+,Ca2+,F−,O2−
How do you find ions on the periodic table?
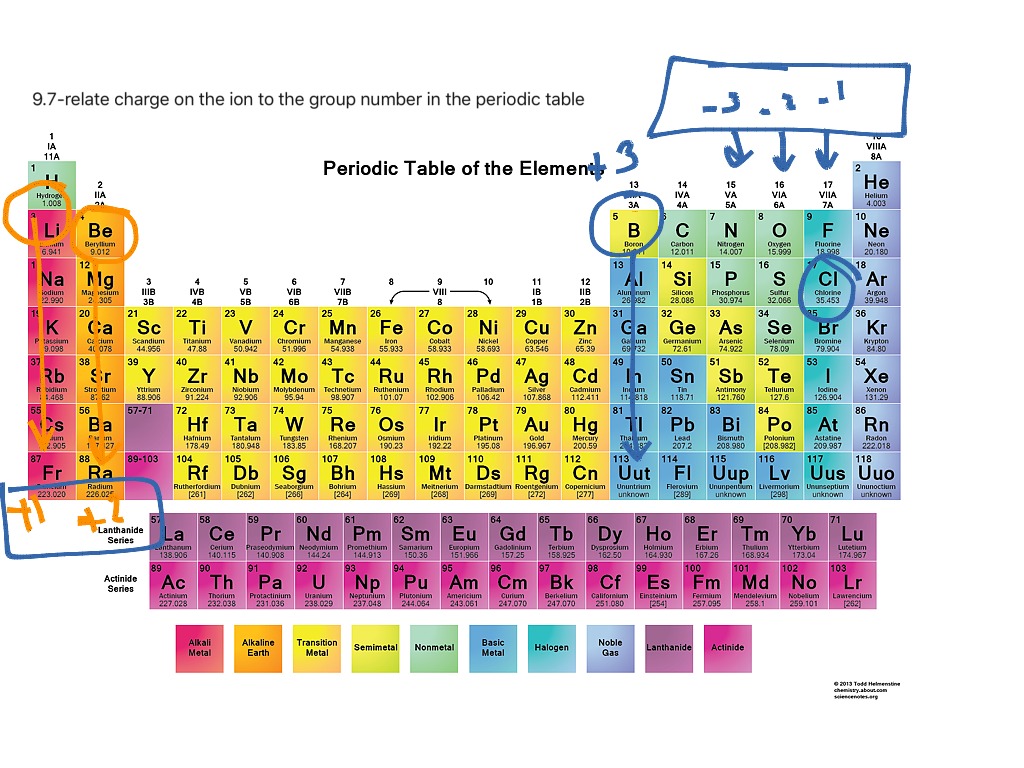
0:001:41Finding the Ionic Charge for Elements on the Periodic Table - YouTubeYouTubeStart of suggested clipEnd of suggested clipAlso called 14 has a plus or minus 4 ionic charge once you reach that plus or minus 4 you start backMoreAlso called 14 has a plus or minus 4 ionic charge once you reach that plus or minus 4 you start back down you have negative 3 negative 2 negative 1. And you should end up with 0 for Group 8a or 18.
What makes an element an ion?
An ion is a charged atom or molecule. It is charged because the number of electrons do not equal the number of protons in the atom or molecule. An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom.
What is an ion simple terms?
1 : an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons.
What elements form 2+ ions?
The alkaline earth metals (red) always form +2 ions. The halogens (blue) always form -1 ions. The calcogens (green) form -2 ions. Many of the transition metals (orange) can have more than one charge.
How are ions formed?
Ions are formed when the number of protons in an atom does not equal the number of electrons. An ion therefore is an atom that has gained or lost one or more electrons and therefore has a negative or positive charge. Ionization is the process of exchanging electrons among atoms or molecules.
What is ion and electron?
An ion (/ˈaɪ.ɒn, -ən/) is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention.
Is an ion still an atom?
Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. An atom can be an ion, but not all ions are atoms.
What is difference between ion and atom?
Atoms vs. Ions. Atoms are neutral; they contain the same number of protons as electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
How do you identify an ion?
The charge of the element should always be represented beside the symbol if it is an ion. For example; sodium and chloride ions are written as Na+ and Cl-, respectively. Refer to an ion with a positive charge as a "cation" and an ion with a negative charge as an "anion."
Why does ion mean?
“I don'tThis ion means “I don't.” It is a spelling based on the colloquial pronunciation of I don't, especially in Black English. Try this: say “I don't know” fast and casually. You're probably saying something that actually sounds like ion–oh. This is actually how we talk, people.
What are three examples of ions?
Many normal substances exist in the body as ions. Common examples include sodium, potassium, calcium, chloride, and bicarbonate. These substances are known as electrolytes. Ions can be created using radiation such as x-rays.
How do you know the charge of an ion?
There is a quick way to work out what the charge on an ion should be: the number of charges on an ion formed by a metal is equal to the group number of the metal. the number of charges on an ion formed by a non-metal is equal to the group number minus eight.
How do you find the ionic formula?
To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its symbol and charge. Finally, combine the two ions to form an electrically neutral compound.
Which group forms ions?
Group 1 atoms lose an electron to form ions with a one positive charge (1+). Group 2 atoms lose two electrons to form ions with a two positive charge (2+). Group 18 (noble gases) normally do not form ions at all. Group 17 often gain one electron to form a negative ion (1- ).
What element forms a 1 ion?
The alkali metals will lose an electron to resemble the next lowest noble gas; thus, all the alkali metals form +1 ions.
How many types of ions are there?
There are only two types of ions- Cations and anions. Cations are positively charged particles or ions and anions are negatively charged particles...
How do you determine ionic charge?
There is a quick way to calculate the charge on an ion: the number of charges on an ion formed by a metal is equal to the metal’s group number. The...
How do you read an ion?
When writing an ion symbol, the one- or two-letter element symbol comes first, followed by a superscript. The superscript indicates the number of c...
Which group in the periodic table cannot form ions?
Since the Group 8A elements have a full octet of eight valence electrons in their highest-energy orbitals, they have a low tendency to gain or lose...
Can ions exist on their own?
Ions of like charge repel each other, and ions of opposite charge attract each other. Therefore, ions do not usually exist on their own but will bi...
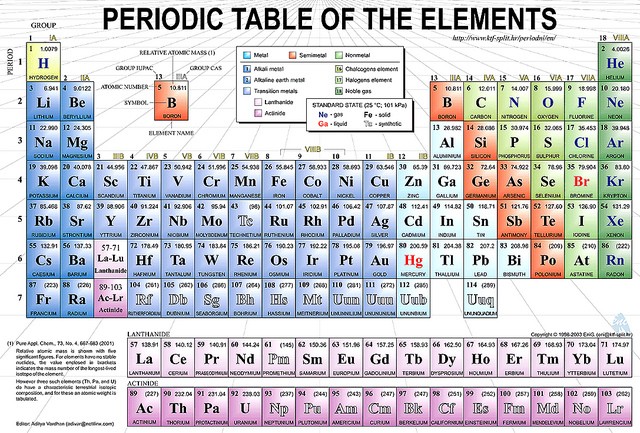
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
Why don't we see ionic charges on rare earths?
One of the reasons you don't normally see a table with charges is because the organization of the table offers a clue to common charges, plus elements can have just about any charge given enough energy and the right conditions.
What is the most requested periodic table?
Updated January 05, 2019. The most-requested printable periodic table has been one for element charges, to predict compounds and chemical reactions. Now, you can use periodic table trends to predict the most common element charges.
Does hydrogen carry ionic charges?
Just keep in mind elements may carry other charges. For example, hydrogen can carry -1 in addition to +1. The octet rule doesn't always apply to ionic charges. In some cases, the charge can exceed +8 or -8! I've got a huge collection of printable periodic tables, which include all 118 elements.
What is the charge generated by an ion?
This electric charge generated on the ion is known as Ionic charge. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed.
What is the ionic charge of an atom?
Ionic Charges of All Elements (List) Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is known as Ionic charge. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, ...
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How many protons does iron have?
Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure. The chemical symbol for Iron is Fe.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
What are the two forces that make up the nucleus?
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
What is the charge of an atom?
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
What is the Pauli exclusion principle?
It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. This fact has key implications for the building up of the periodic table of elements.
How are atoms determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
What type of bonds are formed when electrons are shared or transferred?
Ionic bonds formed with electrons shared or transferred?
Which elements are arranged based on their size from largest to smallest?
Arrange the following elements based on their size from largest to smallest: Ca, Ge, Br, K, Kr
Is matter created or destroyed in a chemical reaction?
In a chemical reaction the mass of the reactants wll be the same as the mass of the products. Matter is not created or destroyed.