What is the carbon group on the periodic table?
carbon group element, any of the six chemical elements that make up Group 14 (IVa) of the periodic table—namely, carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl).
What are facts about the periodic table?
Fun facts about the Periodic Table
- Carbon is unique in that it is known to form up to 10 million different compounds. ...
- Francium is the rarest element on earth. ...
- The only letter not in the periodic table is the letter J.
- The country Argentina is named after the element silver (symbol Ag) which is argentum in Latin.
What are the elements on the periodic table?
hydrogen lithium sodium potassium beryllium magnesium calcium scandium titanium rubidium cesium francium strontium yttrium barium radium zirconium hafnium rutherfordium vanadium niobium tantalum dubnium chromium molybdenum tungsten seaborgium manganese technetium rhenium bohrium iron ruthenium osmium hassium cobalt rhodium iridium meitnerium …
What is labeled on the periodic table?
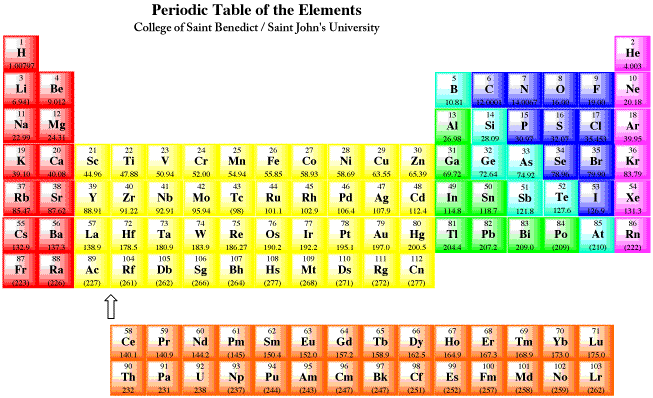
What is labeled on the periodic table? Helium, Neon, Argon , Krypton , Xenon and Radon are six noble gases, found in the periodic table. Given below is a labeled periodic table of elements with their names and atomic number. Hold the mouse on each atomic symbol to know the name of the chemical element. What are the elements of the periodic table?

What is a vertical column in the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table.
Where is cobalt found?
Cobalt is found in the minerals cobaltite, skutterudite and erythrite. Important ore deposits are found in DR Congo, Canada, Australia, Zambia and Brazil.
Why is cobalt important?
Because of its impressive properties cobalt is an important component in wear resistant and corrosive resistant alloys. And cobalt alloys and coatings are seen everywhere from drills to saws, from aircraft turbines to prosthetic bone replacements.
Why is cobalt used in electroplating?
Cobalt metal is sometimes used in electroplating because of its attractive appearance, hardness and resistance to corrosion. Cobalt salts have been used for centuries to produce brilliant blue colours in paint, porcelain, glass, pottery and enamels.
How are elements organized into blocks?
Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The number of protons in an atom.
When was cobalt first used?
Cobalt has been known and used by people for its beautiful colouring and pigment properties as far back as 2500BC. Egyptian cobalt blue paints and Prussian cobalt oxide necklaces have been dated back to this time while cobalt glass has been found in a Greek vase dated at 100 BC.
Is cobalt a trace element?
Cobalt is an essential trace element, and forms part of the active site of vitamin B12. The amount we need is very small, and the body contains only about 1 milligram. Cobalt salts can be given to certain animals in small doses to correct mineral deficiencies. In large doses cobalt is carcinogenic.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is 60 Co?
60 Co (and sometimes 57 Co and 58 Co, with half-lives of 0.75 year and 71 days, respectively) is the key component of the Schilling test, which is a method for determining whether a patient’s body is making and using vitamin B12 properly. The cobalt isotope is used to label cobalt in vitamin B12 to monitor how the body processes this essential vitamin [224].
What are the colors of cobalt?
Some of these compounds are known as: cobalt blue, ceruleum, new blue, smalt, cobalt yellow and cobalt green. In addition to being used as a dye, cobalt is also important to human nutrition as it is an essential part of vitamin B 12.
Why is cobalt used in electroplating?
The metal is used in electroplating because of its appearance, hardness, and resistance to oxidation. Cobalt salts have been used for centuries to produce brilliant and permanent blue colors in porcelain, glass, pottery, tiles, and enamels. It is the principal ingredient in Sevre's and Thenard's blue.
What is cobalt used for?
Cobalt is also used to make alloys for jet engines and gas turbines, magnetic steels and some types of stainless steels. Cobalt-60, a radioactive isotope of cobalt, is an important source of gamma rays and is used to treat some forms of cancer and as a medical tracer.
Why is Kobold called Kobold?
The name derives from the German Kobold for "evil spirits" or "goblins", who were superstitiously thought to cause trouble for miners because the mineral contained arsenic that injured their health and the metallic ores did not yield metals when treated with the normal methods.
Where did the name Cobalt come from?
The name derives from the German Kobold for "evil spirits" or "goblins", who were superstitiously thought to cause trouble for miners because the mineral contained arsenic that injured their health and the metallic ores did not yield metals when treated with the normal methods. Cobalt was discovered in 1735 by the Swedish chemist Georg Brandt.
Is cobalt a metal?
Cobalt is a brittle, hard metal, resembling iron and nickel in appearance. It has a metallic permeability of about two thirds that of iron. Cobalt tends to exist as a mixture of two allotropes over a wide temperature range. The transformation is sluggish and accounts in part for the wide variation in reported data on physical properties of cobalt.
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Do elements of the same group have the same number of valence electrons?
In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons.
Who published the first periodic table?
Anne Marie Helmenstine, Ph.D. Updated March 04, 2019. Dmitri Mendeleev published the first periodic table in 1869. He showed that when the elements were ordered according to atomic weight, a pattern resulted where similar properties for elements recurred periodically.
Which side of the periodic table contains elements that are nonmetals?
Most elements are metals. They are found on the lefthand side of the table. The far right side contains the nonmetals, plus hydrogen displays nonmetal characteristics under ordinary conditions. Elements that have some properties of metals and some properties of nonmetals are called metalloids or semimetals.
What are the two sets of elements?
There are two sets of groups. The group A elements are the representative elements , which have s or p sublevels as their outer orbitals. The group B elements are the nonrepresentative elements, which have partly filled d sublevels (the transition elements) or partly filled f sublevels (the lanthanide series and the actinide series ). The Roman numeral and letter designations give the electron configuration for the valence electrons (e.g., the valence electron configuration of a group VA element will be s 2 p 3 with 5 valence electrons).
How are elements in the periodic table arranged?
Elements in the periodic table are arranged in periods (rows) and groups (columns). Each of the seven periods is filled sequentially by atomic number. Groups include elements having the same electron configuration in their outer shell, which results in group elements sharing similar chemical properties. The electrons in the outer shell are termed ...
What are the electrons in the outer shell of an element?
The electrons in the outer shell are termed valence electrons. Valence electrons determine the properties and chemical reactivity of the element and participate in chemical bonding. The Roman numerals found above each group specify the usual number of valence electrons. There are two sets of groups.
What are the recurring properties of the periodic table?
These properties and their trends are: Ionization Energy - energy needed to remove an electron from a gaseous atom or ion. Ionization energy increases moving left to right and decreases moving down an element group (column).
What is the periodic law?
This led to the formulation of the periodic law, which states that the chemical properties of the elements are dependent on their atomic numbers.
What is the term for the charge generated when an atom loses one or more electrons?
Periodic table with Charges Labeled on it (7 HD Images) Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is known as Ionic charge . When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, ...
Can you get a full size periodic table?
You can also get the full size Periodic table with charges of ions, from this article only. (Downloading link given below)
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Which gases have high thermal conductivity?
The mean free path also depends on the diameter of the molecule, with larger molecules more likely to experience collisions than small molecules, which is the average distance traveled by an energy carrier (a molecule) before experiencing a collision. Light gases , such as hydrogen and helium typically have high thermal conductivity. Dense gases such as xenon and dichlorodifluoromethane have low thermal conductivity.
What is the electron configuration of oxygen?
Electron configuration of Oxygen is [He ] 2s2 2p4.
How many protons does oxygen have?
Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. The chemical symbol for Oxygen is O.
What is the atomic radius of oxygen?
The atomic radius of Oxygen atom is 66pm (covalent radius).
What is the electronegativity of oxygen?
The electronegativity of Oxygen is: χ = 3.44
Does thermal conductivity depend on temperature?
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general: