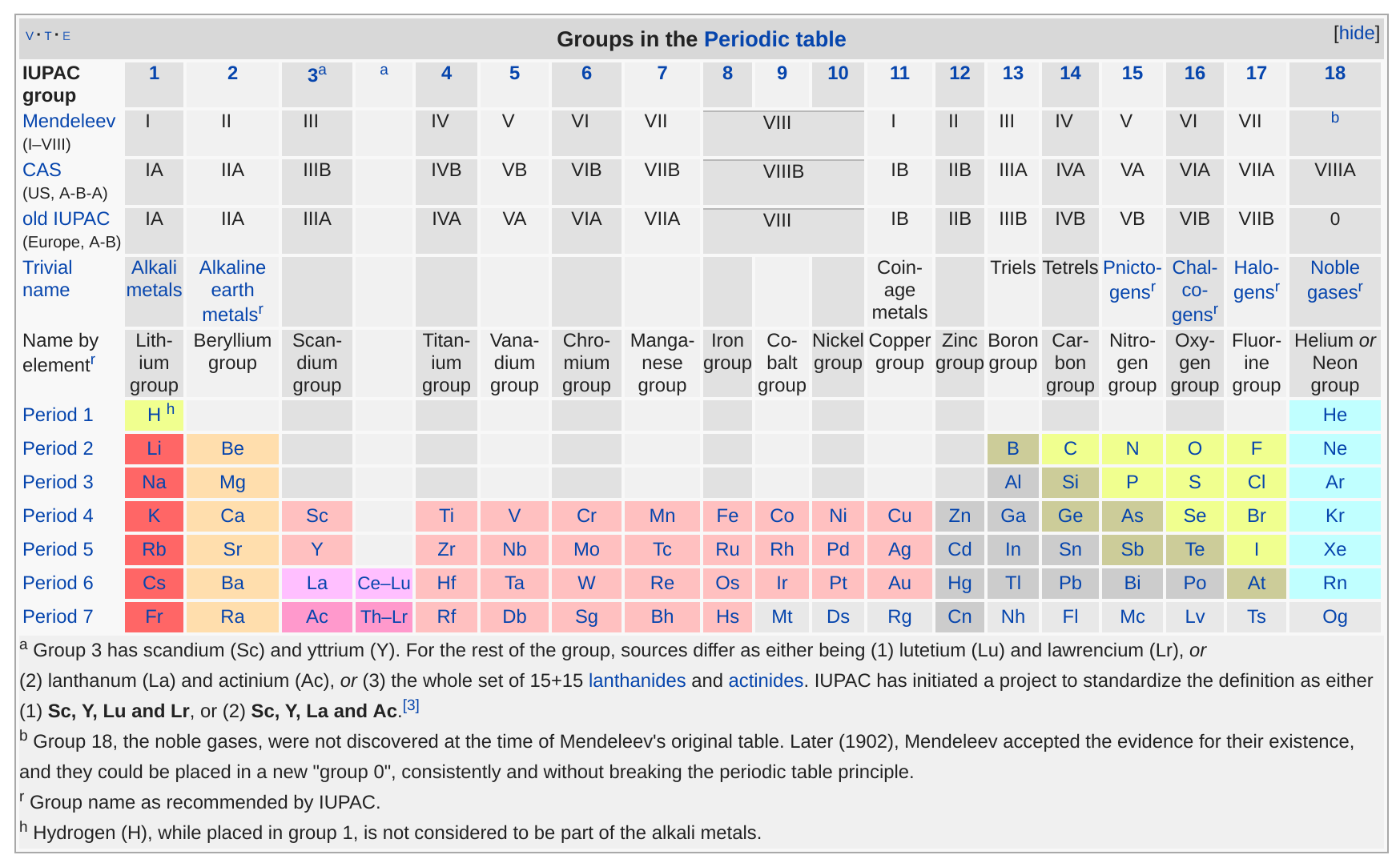
What are the groups on the periodic table called?
- Lithium (Li)
- Sodium (Na)
- Potassium (K)
- Rubidium (Rb)
- Cesium (Cs)
- Francium (Fr)
What are the groups of elements on the periodic table?
Group. See About the Periodic Table for information on how Group can be used to characterize an element. Group 1: alkali metals, or lithium family. Group 2: alkaline earth metals, or beryllium family. Group 3: the scandium family. Group 4: the titanium family. Group 5: the vanadium family. Group 6: the chromium family.
What does group in periodic table mean?
group, in chemistry, a column in the periodic table of the chemical elements. In a group, the chemical elements have atoms with identical valence electron counts and identical valence vacancy counts. This similarity in both the composition and structure of their atomic valence shells implies a corresponding similarity in both their chemical and physical properties.
What are the characteristics of the periodic table?
The characteristics of modern periodic table are:
- The arrangement of elements in modern periodic table is based on their electronic configurations.
- The horizontal rows of elements in periodic table are called periods.
- There are 7 periods in modern periodic table.
- The elements in a period have consecutive atomic numbers. ...
- The vertical columns in a periodic table are called groups.
- There are
Which group of the periodic table is alkaline earth metals in?
What are the elements that are grouped together?
Why are alkaline earth metals so reactive?
What group is alkaline earth metals in?
What group is strontium in?
Which metals form ionic bonds?
Where are alkaline metals found?
See 4 more
About this website

What is the Group 2A element in period 2?
The Group 2A element in period 2 is berylium (Be) b e r y l i u m ( B e ) .
What are properties of Group 2A elements?
Let's quickly review some properties they all share:They have 2 valence electrons, which they can lose, forming a +2 cation.Most form ionic bonds.They are reactive so they are not found in their pure form in nature.They are all found in the earth's crust and form an alkaline solution when added to water.More items...•
Where is group 2A located?
Group 2 elements are located on the left side of the periodic table and are often referred to as alkaline earth metals because they form an alkaline solution with water, are found naturally in the earth, and are metals. This group includes beryllium, magnesium, calcium, strontium, barium, and radium.
What are Group 2 elements called?
alkaline-earth metalalkaline-earth metal, any of the six chemical elements that comprise Group 2 (IIa) of the periodic table. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Is group 2A cation or anion?
Group 1A and 2A of the periodic table, alkali metals and alkaline earth metals respectively, always form cations. In contrast, Group 17A, which consists of halogens, always forms anions.
Why are group 2 called alkaline earth?
They are called "alkaline" earth metals because they form "alkaline" solutions, hydroxides, when they react with water . "Earth" was the alchemists term for the oxides of alkaline earth metals.
Why is group 2A alkaline earth metals?
The elements of group - 2 are called alkaline earth metals because because their oxides and hydroxides are alkaline in nature and these metal oxides are found in the earth's crust.
What is group 3A called?
In the modern periodic table, Group 3A or IIIA is simply called Group 13. Group 3A elements belong to the boron family, which is a series of elements that occupy the 13th column on the periodic table. The reason why the boron family is called by this name is because the first element in Group 3A is boron.
What is group 4A called?
Group 4A or Group 14 is a set of elements of the periodic table known as the Carbon or Carbonoids Group. It is found in the 14th column of the periodic table. Its elements are: Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb), and Flerovium (Fl).
Are Group 2 elements metals or nonmetals?
Group 2 elements are classified as metals. Group 2 elements are also known as alkaline earth metals.
What are Group 2 elements used for?
Compounds of Group 2 elements are used to neutralise acidic conditions. As group 2 elements are alkaline earth metals, they neutralise acids when they react with them. Calcium hydroxide Ca(OH)2 is used in agriculture.
What do the elements of group II 2 have in common?
The property that is common to all group 2 elements is that they tend to form ionic bonds by losing electrons making these atoms positive charge. They are called cations. These elements are beryllium, magnesium, calcium, strontium, and radium.
What do all group 2 elements have in common?
All the elements in Group 2 have two electrons in their valence shells, giving them an oxidation state of +2. Covers the elements beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr) and barium (Ba).
Why do the elements in group IIA have similar properties?
Elements in the same group have similar properties because they have the same number of valence electrons in their outermost shell.
What is the charge for all elements in group 2A?
Thus, the group 2 metals tend to have a +2 charge.
What are the properties of alkaline earth metals?
Key PointsAlkaline earth metals have two valence electrons.They have low ionization energy, low electron affinity, and low electronegativity.They are highly reactive and often form divalent cations.They are good conductors of electricity.Alkaline earth metals have an oxidation state of +2.
What family of elements does group 2 correspond to?
Group 2 of elements on the periodic table refers to alkaline earth metals. They are beryllium, magnesium, calcium, strontium, barium, and radium.
What are the properties of group 2 elements?
Some of the properties of group 2 elements are that they have low boiling points, low melting points, and low densities.
What are some properties of group 2A?
The properties of group 2A elements are that they are fairly reactive. They form cations readily by losing 2 electrons from their outermost shells.
The Parts of the Periodic Table - Angelo State University
Group 2A (or IIA) of the periodic table are the alkaline earth metals: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).They are harder and less reactive than the alkali metals of Group 1A. The name comes from the fact that the oxides of these metals produced basic solutions when dissolved in water, and they remained solids at the temperatures ...
Periodic table Groups Explained !! (With 1-18 Group Names)
Final words. Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
Alkali Metals: Uses and Properties - Study.com
Learn about the alkali metals group and explore the group 1A elements. Understand their charge, valence electrons, ions, and find out whether they...
Why are Group 2 elements called alkaline metals?
Group 2 elements are called alkaline metals because they form alkaline solutions, hydroxides, when reacting with water and their oxides are found in the earth’s crust.
WHAT IS PERIODIC TABLE?
It is a tabular display of chemical elements, arranged in order of atomic number in rows so that elements with similar atomic structure and recurring chemical properties appear in vertical columns. The seven rows of the table are called a periodic table.
What are the different groups of elements?
There are multiple ways of grouping the elements but they are commonly divided into metals, non-metals, metalloids. There are also more specific groups like alkali metals, transition metals, rare metals, alkaline earth, halogens, and noble gases. The 18 vertical columns of the table are called Groups. The s-, p- and d-block elements of the periodic ...
How many rows are there in the periodic table?
The seven rows of the table are called a periodic table. In the periodic table, chemical elements are present in rows horizontally in order of increasing atomic number and vertically according to the recurring properties of their atoms. Metals are on the left side and non-metal are on the right side of the periodic table.
Is barium a metal?
Barium is a very reactive metallic element. It resembles calcium. It is a silvery-white metal. When it cuts, it quickly turns into a black color due to the formation of barium oxide. Barium is commonly found as barite and witherite. It has a good conductivity.it is a mixture of 6 stable isotopes. Barium compounds that are soluble having a very poisonous property.
Is strontium a non-toxic element?
Strontium isotopes are considered non-toxic. Strontium is a mixture of 4 stable isotopes. It a silvery metal with a pale yellow tint. It is harder than barium and softer than calcium. When strontium reacts with water it produces strontium hydroxide and hydrogen gas. It occurs naturally in compounds and other elements that’s why it has extreme reactivity with oxygen and air.
What is Group 2 metal?
Group 2 elements are often referred to as the alkaline earth metals because. they form an alkaline (or basic) solution when they are combined with water. they can be found naturally in the earth. they are metals.
How many valence electrons are in a group 2?
All group 2 elements have two valence electrons, or the electrons furthest from the nucleus, which makes them reactive, meaning the elements want to combine with other elements. Group 2 elements can be found in a lot of products for various uses. Let's review:
What is the material that is made of group 2 elements?
This causes the gypsum to lose the water thus becoming plaster of Paris. Let's move onto a third important material made from group 2 elements: cement, used in the construction of sidewalks, bridges, and giant skyscrapers. Like the quicklime and plaster of Paris, cement uses calcium.
What is the group 2 element in Quicklime?
Oxides are formed when an element reacts with oxygen. Quicklime is one such oxide, specifically, calcium oxide. It's been used for centuries, dating back to the ancient Romans, Greeks and Egyptians. Today it's used in the manufacturing of numerous goods such as varnish, paper, soap, and rubber. It is also used as plaster and mortar. What's the group 2 element in quicklime? If you check out the periodic table, you will see that calcium is a group 2 element.
What does the IIA above be mean?
Note the IIA above Be. This indicates this column is group 2 elements
Which group of elements has two valence electrons?
This group includes beryllium, magnesium, calcium, strontium, barium, and radium. Being in group 2 means they all have two valence electrons, or the electrons that are furthest away from the nucleus. These valence electrons are typically the electrons involved in reactions.
Which group of elements are found naturally in the Earth?
Lesson Summary. Group 2 elements are located on the left side of the periodic table and are often referred to as alkaline earth metals because they form an alkaline solution with water, are found naturally in the earth, and are metals. This group includes beryllium, magnesium, calcium, strontium, barium, and radium.
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
What is Group 2A?
Group 2A (or IIA) of the periodic table are the alkaline earth metals : beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They are harder and less reactive than the alkali metals of Group 1A. The name comes from the fact that the oxides of these metals produced basic solutions when dissolved in water, ...
How many electrons does an alkaline metal have?
The alkaline earth metals have two valence electrons in their highest-energy orbitals ( ns2 ). They are smaller than the alkali metals of the same period, and therefore have higher ionization energies. In most cases, the alkaline earth metals are ionized to form a 2+ charge.
Which metals have a higher melting point than alkali metals?
In most cases, the alkaline earth metals are ionized to form a 2+ charge. The alkaline earth metals have much higher melting points than the alkali metals: beryllium melts at 1287ºC, magnesium at 649ºC, calcium at 839ºC, strontium at 768ºC, barium at 727ºC, and radium at 700ºC. They are harder metals than the Group 1A elements, ...
Why are alkaline earth metals so reactive?
Like the Group 1A elements, the alkaline earth metals are too reactive to be found in nature in their elemental form.
Where is the element radius found?
It is found in the Earth's crust at a concentration of 0.6 ppt (parts per trillion), making it the 84th most abundant element.
Is Group 2A salt soluble in water?
They are harder metals than the Group 1A elements, but are soft and lightweight compared to many of the transition metals. Salts of the Group 2A metals are less soluble in water than those of Group 1A because of the higher charge densities on the 2+ cations; nevertheless, many Group 2A salts are at least moderately soluble.
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
What are the elements that chemists classify?
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
Which group of the periodic table is alkaline earth metals in?
Some alkaline earth metals run through your veins, whereas others make you extremely sick. They make up six elements and all belong to group 2A on the left of the periodic table.
What are the elements that are grouped together?
Alkaline earth metals include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). Elements are grouped together because they share similar properties. Now you might be thinking, a radioactive element shares properties with the calcium in my milk?!
Why are alkaline earth metals so reactive?
Due, in part, to those two valence electrons, alkaline earth metals are extremely reactive. This means they want to bond or attach to other elements. Although they aren't as reactive as their neighbors to the left, the alkali metals, they're still pretty reactive. Because they are so reactive, you can't find alkaline earth metals in their pure form in nature.
What group is alkaline earth metals in?
Alkaline earth metals make up group 2A on the periodic table. Explore the definition of alkaline earth metals, their shared properties, and how they affect human health both negatively and positively. Updated: 11/01/2021
What group is strontium in?
Strontium is one of six elements belonging to the alkaline earth metals, or group 2A. Groups are the vertical columns on a periodic table, and group 2A is on the left. Sometimes, group 2A is represented by roman numerals, or IIA.
Which metals form ionic bonds?
Most of the alkaline earth metals form ionic bonds where valence electrons are transferred from one atom to another, but beryllium and magnesium can also form covalent bonds, where electrons are shared between atoms.
Where are alkaline metals found?
They are all found in the earth's crust and form an alkaline solution when added to water. Both of these facts should help you with the name - alkaline earth metals.
