Does 'H' stand for hydrogen on the periodic table?
Hydrogen is a nonmetal and is placed above group in the periodic table because it has ns 1 electron configuration like the alkali metals. However, it varies greatly from the alkali metals as it forms cations (H + ) more reluctantly than the other alkali metals .
What does he stand for on the periodic table?
What does He stand for in the periodic table . H. + Abbreviation for hydrogen ion, the proton. 300. Who named the periodic table? 2022 - Question & Answers Phosphorus . How many protons, neutrons, and electrons? HAGER!!! The symbol "B" on the periodic table stands for Boron. From natrium, a synonym for sodium.
What does HF stand for on the periodic table?
What Does Au Stand For On The Periodic Table? Published by: Agueda Brown | Date created: May 9, 2021. Gold. Atomic Names and Symbols. Atomic Number Symbol Name; 79: Au: Gold: 72: Hf: Hafnium: 108: Hs: Hassium: 2: He: Helium: Similar Questions. What does Au mean in the periodic table? Gold Gold is element 79 and its symbol is Au. Though the name ...
What is element 59 on the periodic table?
She has taught science courses at the high school, college, and graduate levels. Praseodymium is element 59 on the periodic table with the element symbol Pr. It's one of the rare earth metals or lanthanides. Here is a collection of interesting facts about praseodymium, including its history, properties, uses, and sources.
See more
Is hydrogen H or H2?
Hydrogen has a molar mass of 1 and it's molecular formula is H2. Hydrogen, H, is the lightest element with the atomic number 1. It is a colorless, odorless, tasteless, and highly flammable gas with the molecular formula H2.
Where is H on the periodic table?
What is the Position of Hydrogen in the Periodic Table? Hydrogen is the first element of the periodic table as its atomic number is one, which means it has only one electron in its atom and thus only one electron is present in its outermost shell.
Is H element a metal?
In brief explanation, Hydrogen is a nonmetal and is placed above group in the periodic table because it has ns1 electron configuration like the alkali metals.
What family is H in on the periodic table?
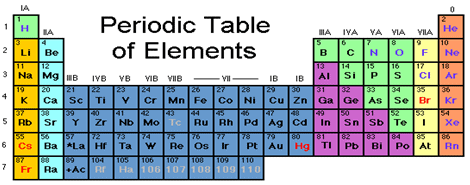
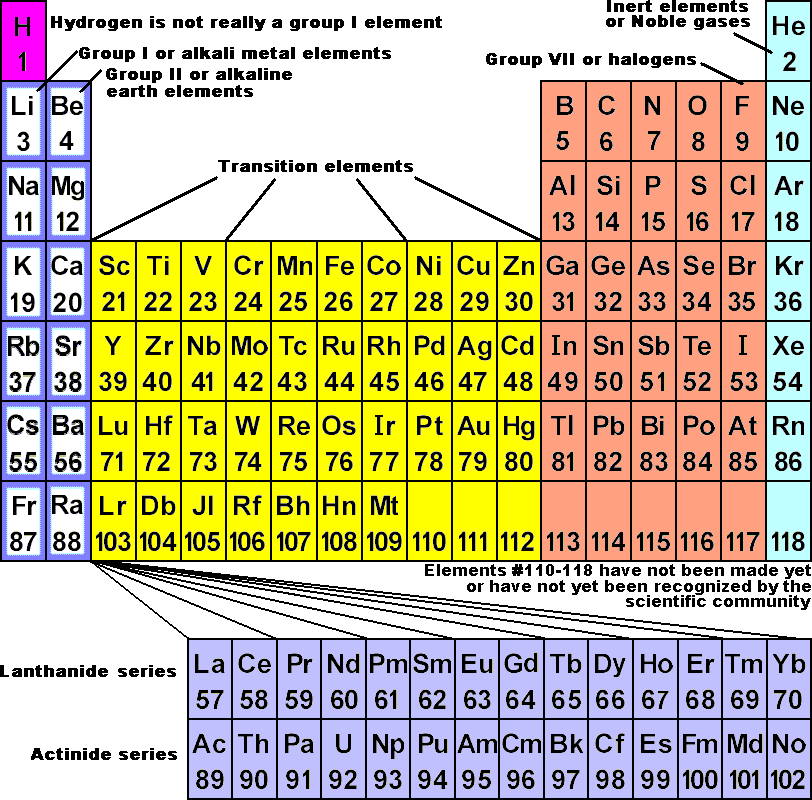
Group 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These are (except for hydrogen) soft, shiny, low-melting, highly reactive metals, which tarnish when exposed to air.
What are 5 uses of hydrogen?
Hydrogen is used in many industrial processes. ... Hydrogen is used for exploring outer space. ... Hydrogen fuel cells produce electricity. ... Burning hydrogen for electricity generation. ... Hydrogen use in vehicles.
Why is hydrogen so reactive?
Group 1 of the periodic table includes hydrogen and the alkali metals. Because they have just one valence electron, group 1 elements are very reactive.
Why is hydrogen a nonmetal?
Answer: Because hydrogen has the same ns1 electron configuration as the alkali metals, hydrogen is classified as a non-metal and is positioned above group in the periodic chart. It differs from the other alkali metals in that it creates cations (H+) more reluctantly than the others.
Why is hydrogen so important?
Hydrogen can be used to power vehicles, generate electricity, power industry and heat our homes and businesses. It could make a huge difference on our carbon emissions, and will be critical to achieving net zero.
What is hydrogen made of?
Hydrogen can be produced from a variety of domestic resources, such as natural gas, nuclear power, biomass, and renewable power like solar and wind. These qualities make it an attractive fuel option for transportation and electricity generation applications.
What are halogens known for?
What are the major properties of the halogen elements? Halogen elements are very reactive. With sodium, they produce salts, of which table salt (sodium chloride, NaCl) is the most well known. Each halogen atom has seven valence electrons in its outermost electron shell.
Is hydrogen a halogen?
It can come under a halogen as it shows properties similar to them,where as, it can come under an alkaline metal as it has only one electron in its valence shell. So hydrogen is a halogen and alkaline metal.
Why is hydrogen on its own in the periodic table?
Because hydrogen has an atomic number of one, it has only one electron in its atom and consequently only one electron in its outermost shell, making it the first element in the periodic table. The electrical configuration of elements determines their placement in the periodic table.
What are the 7 periods in periodic table?
Period 7 elementHydrogenHeliumLithiumBerylliumNeonSodiumMagnesiumArgonPotassiumCalciumKryptonRubidiumStrontiumXenon2 more rows
What is the name of element H3?
Triatomic hydrogenIdentifiersChemical formulaH3Molar mass3.024 g·mol−1Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references6 more rows
What is the special name of group 17?
halogenhalogen, any of the six nonmetallic elements that constitute Group 17 (Group VIIa) of the periodic table. The halogen elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts).
What are the 2 rows At the bottom of the periodic table?
The two rows of 14 elements at the bottom of the periodic table are the lanthanides and the actinides, whose positions in the periodic table are indicated in group 3.
Where is hydrogen in the periodic table?
Hydrogen element is in group 1 and period 1 of the Periodic table. Hydrogen is placed along with the alkali metals group as it has the similar outer electron configuration as that of the group 1 elements (i.e ns1).
How many electrons are in a hydrogen atom?
Now in the Hydrogen atom, there is only one proton and only one electron.
Why is hydrogen gas used in balloons?
Hence due to its light-weight, it always moves upward in the atmosphere. Because of the light weight of hydrogen gas, it is also used in party balloons.
What is the mass of an atom?
Explanation: The mass of an atom completely depends on the number of protons and number of neutrons present in the nucleus of an atom. Protons and neutrons are the heavy particles in an atom. The mass of protons and neutrons is the same and it is 1.6749 × 10 -27 kg.
How much hydrogen is in the air?
There is around 0.000055 % of hydrogen in the air.
Why is hydrogen in the 1st group?
Apart from having similar properties as that of group 1 and group 17 elements, hydrogen element is placed in 1st group because it has similar outermost electron configuration as that of alkali metals (i.e ns1).
Which configuration of an atom should be used for stability?
The atom should have duplet or octet configuration for its stability.
What is a vertical column in the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table.
Where is hydrogen found in the universe?
Natural abundance. Hydrogen is easily the most abundant element in the universe. It is found in the sun and most of the stars, and the planet Jupiter is composed mostly of hydrogen. On Earth, hydrogen is found in the greatest quantities as water.
How is hydrogen produced?
Any hydrogen that does enter the atmosphere quickly escapes the Earth’s gravity into outer space. Most hydrogen is produced by heating natural gas with steam to form syngas (a mixture of hydrogen and carbon monoxide). The syngas is separated to give hydrogen. Hydrogen can also be produced by the electrolysis of water.
What is hydrogen used for?
In the chemical industry it is used to make ammonia for agricultural fertiliser (the Haber process) and cyclohexane and methanol, which are intermediates in the production of plastics and pharmaceuticals. It is also used to remove sulfur from fuels during the oil-refining process.
What is the oxidation state of an atom?
The oxidation state of an atom is a measure of the degree of oxidation of an atom. It is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an oxidation state of 0. The sum of the oxidation states within a compound or ion must equal the overall charge.
Is hydrogen gas a clean fuel?
Some see hydrogen gas as the clean fuel of the future – generated from water and returning to water when it is oxidised. Hydrogen-powered fuel cells are increasingly being seen as ‘pollution-free’ sources of energy and are now being used in some buses and cars. Hydrogen also has many other uses.
Who discovered that hydrogen is flammable?
In the early 1500s the alchemist Paracelsus noted that the bubbles given off when iron filings were added to sulfuric acid were flammable. In 1671 Robert Boyle made the same observation. Neither followed up their discovery of hydrogen, and so Henry Cavendish gets the credit. In 1766 he collected the bubbles and showed that they were different from other gases. He later showed that when hydrogen burns it forms water, thereby ending the belief that water was an element. The gas was given its name hydro-gen, meaning water-former, by Antoine Lavoisier.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).