What are 5 facts about Iron?
Top 10 Facts about The Iron Age
- The Iron Age was between 500 BC to 43 AD in Britain. The date range of the Iron Age varied across the world. ...
- Iron became popular and was what was mainly used during this time. During the Iron Age, iron became the most popular material to use when making tools and weapons. ...
- People were ruled by warrior Kings! ...
- Iron was used to make sharp objects! ...
What are the main metals in the periodic table?
The six alkaline earth metals are:
- beryllium
- magnesium
- calcium
- strontium
- barium
- radium
What is 57 on the periodic table?
The atomic number of Lanthanum is 57 and it is the first element of the lanthanide series. So,La is the 57th element of the periodic table. Lanthanum. What is the 57th element of the periodic table? What is the first element of the periodic table? Originally Answered: Can you tell the first 10 elements of the periodic table of elements? 2.
What is ferrous on the periodic table?
The periodic table on the left separates elements into three groups: the metals (green in the table), nonmetals (orange), and metalloids (blue). Most elements are metals. They are usually shiny, very dense, and only melt at high temperatures. ... Ferrous metals are metals that consist mostly of iron and small amounts of other elements.

What is iron on the periodic?
Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. Iron is a group 6 and period 4 metal.
Why is iron called Fe?
The Latin name for iron is ferrum, which is the source of its atomic symbol, Fe. The word iron is from an Anglo-Saxon word, iren. The word iron is possibly derived from earlier words meaning "holy metal" because it was used to make the swords used in the Crusades, according to WebElements.
What group is iron in periodic table?
Group 8iron (Fe), chemical element, metal of Group 8 (VIIIb) of the periodic table, the most-used and cheapest metal.
What period is iron on the periodic table?
Iron is the 26th element on the periodic table. It is located in period 4 and group 8.
What are 5 interesting facts about iron?
Read on to find out why we think iron's so wonderful!It's the Sixth Most Common Element in the Universe. ... Iron Is Vital for the Human Body. ... It Was Originally Called Ferrum. ... The First Workable Iron Came from Outer Space. ... The Eiffel Tower's Made from Wrought Iron. ... Steel Is Iron Too. ... It's Incredibly Strong.More items...
Why is the element iron so important?
The element iron is an essential mineral for all living organisms. In animals, iron is used to make hemoglobin, a protein in red blood cells responsible for the transportation of oxygen from the lungs to the rest of the body. Iron is also present in myoglobin, the muscle cells which stores and transports oxygen.
What type of metal is iron?
Iron (/ˈaɪərn/) is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table.
What is Group 7 called?
The Group 7 elements are called the halogens. They are placed in the vertical column, second from the right, in the periodic table . Chlorine, bromine and iodine are the three common Group 7 elements. Group 7 elements form salts when they react with metals. The term 'halogen' means 'salt former'.
Is iron a main group element?
Group 8 is a group (column) of chemical elements in the periodic table. It consists of iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs). They are all transition metals....Group 8 element.IronCopperZincGalliumGermanium6 more columns
Why iron is a metal?
Iron is called metal because it is malleable and ductile and has high density and hard.
What elements make up iron?
It contains at least 10.5% chromium. Other metals such as nickel, molybdenum, titanium and copper are added to enhance its strength and workability. It is used in architecture, bearings, cutlery, surgical instruments and jewellery. Cast iron contains 3–5% carbon.
What's made of iron?
Vehicles – cars, trucks, SUVs, semis, RVs, buses, trains. Appliances – refrigerators, washing machines, clothes dryers, stoves, dishwasher. Utensils – forks, spoons, knives and more. Medical – surgical stainless steel, implantable devices.
What is the scientific name of iron?
The chemical symbol of iron is 'Fe. ' which comes from the Latin word that is 'Ferrum'. There are many words in science that are derived from Latin and Greek languages. Therefore, the term 'Ferrum' is the chemical name of the element iron. Hence, the chemical name of the iron is Ferrum.
What is the Latin name of iron?
FerrumIron (Fe – Ferrum)
Why does iron only have 14 electrons in the third shell?
To do so, it will try to fill up its 3rd shell or M Shell. The M Shell can contain maximum 8 electrons but in iron it contains : 3s2 3p6 3d6. That is, 2+6+6=14 electrons.
What block is Fe?
dFact boxGroup81538°C, 2800°F, 1811 KBlockd7.87Atomic number2655.845State at 20°CSolid56FeElectron configuration[Ar] 3d64s27439-89-62 more rows
Iron in Periodic table
Iron element is in group 8 and period 4 of the Periodic table. Iron is the d-block element and it belongs to transition metals group.
Is Iron a Transition Metal? Why?
Yes, Iron is a transition metal because it has incompletely filled d-orbital in its ground state.
Properties of Iron
The physical and chemical properties of Iron element are mentioned below.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
Where did iron come from?
Iron objects have been found in Egypt dating from around 3500 BC. They contain about 7.5% nickel, which indicates that they were of meteoric origin.
How is iron made?
Commercially, iron is produced in a blast furnace by heating haematite or magnetite with coke (carbon) and limestone (calcium carbonate). This forms pig iron, which contains about 3% carbon and other impurities, but is used to make steel. Around 1.3 billion tonnes of crude steel are produced worldwide each year.
Why did microbes develop soluble siderophore molecules?
Surviving terrestrial and ocean-dwelling microbes developed soluble siderophore molecules to regain access to this plentiful, but otherwise inaccessible essential resource, which used hydroxamate or catechol chelating groups to bring the FeIII back into solution. Eventually higher organisms including animals, evolved. And animals used the energy of oxygen recombining with the hydrocarbons and carbohydrates in plant life to enable motion. Iron was essential to this process.
What is ordinary carbon steel?
Ordinary carbon steel is an alloy of iron with carbon (from 0.1% for mild steel up to 2% for high carbon steels), with small amounts of other elements. Alloy steels are carbon steels with other additives such as nickel, chromium, vanadium, tungsten and manganese.
How much iron is in the human body?
Iron is an essential element for all forms of life and is non-toxic. The average human contains about 4 grams of iron. A lot of this is in haemoglobin, in the blood. Haemoglobin carries oxygen from our lungs to the cells, where it is needed for tissue respiration. Humans need 10–18 milligrams of iron each day.
How are elements organized into blocks?
Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The number of protons in an atom.
What is a vertical column in the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Period. A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. Block.
What is the name of the mineral that contains iron?
Iron pyrite (iron disulfide, FeS 2) is a crystalline gold-colored mineral known as fool’s gold. Chromite is a chromium ore that contains iron. Lodestone is a form of magnetite that exhibits natural magnetic properties [3].
How many isotopes are in iron?
Naturally occurring iron consists of four isotopes: 5.85 percent of slightly radioactive 54Fe (half-life >3.1×1022 years), 91.75 percent of stable 56Fe, 2.12 percent of stable 57Fe, and 0.28 percent of stable 58Fe. In addition, it appears that the naturally occurring radioactive isotope 60Fe, with a half-life of 1.5 million years, is now extinct, but it can be produced synthetically. Much of the past work on measuring the isotopic composition of iron centered on determining 60Fe variations due to processes accompanying nucleosynthesis (that is, through meteorite studies) and ore formation.
What is iron oxide?
Iron (III) oxide: ferric oxide, or red iron oxide (Fe2O3): This compound corresponds to iron rust. Its mineral form, known as hematite, is mined as the main ore of iron and is used in the production of iron in a blast furnace.
What is ferrous sulphate used for?
Ferrous sulphate is used to treat anemia (iron-deficiency). The most common use of iron is in manufacturing of steel, that has various attractive properties and uses. Cast iron (with 3-5% carbon) is used for making pumps, pipes and valves. Iron and steel are widely used in civil engineering and construction.
What is the oxidation state of iron?
Like the other group 8 elements, iron exists in a wide range of oxidation states, −2 to +7, although +2 and +3 are the most common. Fresh iron surfaces appear lustrous silvery-gray, but oxidize in normal air to give hydrated iron oxides, commonly known as rust.
What is the element with the symbol Fe?
Iron. Iron is a chemical element with symbol Fe and atomic number 26. It is a metal in the first transition series. It is by mass the most common element on Earth, forming much of Earth’s outer and inner core. It is the fourth most common element in the Earth’s crust.
Why is iron abundant in rocky planets?
Its abundance in rocky planets like Earth is due to its abundant production by fusion in high-mass stars, where it is the last element to be produced with release of energy before the violent collapse of a supernova, which scatters the iron into space.
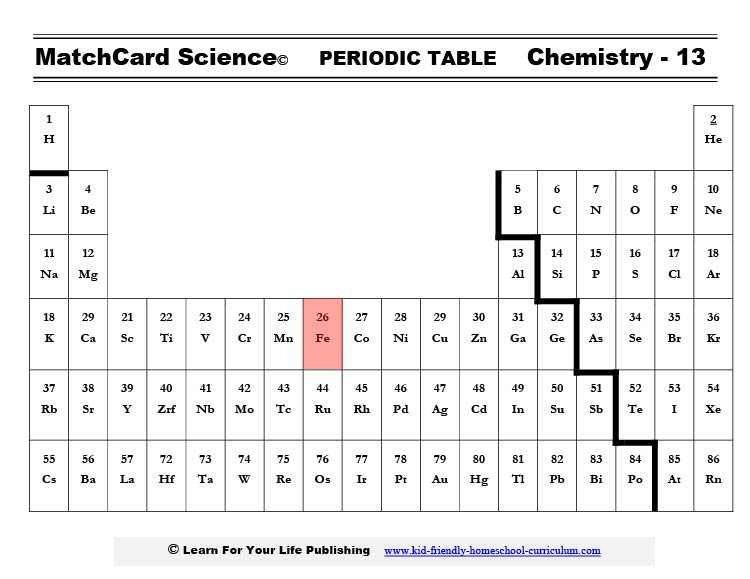
Where Is Iron Found On The Periodic Table?
Iron's location in the periodic table of the elements. Todd Helmenstine
What are homologous elements?
Homologous elements are those found in the same group of the periodic table. They share electrochemical properties with one another. Homologues of iron are ruthenium, osmium, and hassium. Cite this Article. Format. mla apa chicago. Your Citation. Helmenstine, Anne Marie, Ph.D.
How many protons does iron have?
Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure. The chemical symbol for Iron is Fe.
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
How many protons does phosphorus have?
Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure. The chemical symbol for Phosphorus is P.
How many electrons does a scandium have?
Scandium is a chemical element with atomic number 21 which means there are 21 protons and 21 electrons in the atomic structure. The chemical symbol for Scandium is Sc.
How are the chemical properties of a solid, liquid, gas, and plasma determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
What is the temperature of nitrogen?
Liquid nitrogen (made by distilling liquid air) boils at 77.4 kelvins (−195.8°C) and is used as a coolant.
What is the most common type of boron?
There are over 100 different borate minerals, but the most common are: borax , kernite, ulexite etc. Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Do elements of the same group have the same number of valence electrons?
In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons.

Occurrence
Physical Properties
- The Latin name for iron is ferrum, which is the source of its atomic symbol, Fe atomic number 26; atomic weight 55.845; melting point about 1,535°C; boiling point about 2,750°C and specific gravity of 7.87 at 20°C. Like the other group 8 elements, iron exists in a wide range of oxidation states, −2 to +7, although +2 and +3 are the most common. Fresh iron surfaces appear lustrous …
Chemical Characteristics
- In chemical terms, it is classified as a transition metal. It is located in period 4 of the periodic table, situated between manganese and cobalt. In addition, it lies at the top of group 8 (former group 8B). Iron, cobalt, and nickel have a number of similar properties and were once grouped together as group 8B . Iron has the ability to form variab...
Significance and Uses
- Iron (III) oxide: ferric oxide, or red iron oxide (Fe2O3): This compound corresponds to iron rust. Its mineral form, known as hematite, is mined as the main ore of iron and is used in the productio...
- Iron (III) oxide-hydroxide, hydrated iron oxide, or yellow iron oxide (FeO(OH)): This solid material has colors ranging from yellow through dark brown to black. It occurs naturally as m…
- Iron (III) oxide: ferric oxide, or red iron oxide (Fe2O3): This compound corresponds to iron rust. Its mineral form, known as hematite, is mined as the main ore of iron and is used in the productio...
- Iron (III) oxide-hydroxide, hydrated iron oxide, or yellow iron oxide (FeO(OH)): This solid material has colors ranging from yellow through dark brown to black. It occurs naturally as minerals goet...
- Iron (II) sulfate or ferrous sulfate (FeSO4): It is commonly used additive that is found in various foods. Ferrous sulphate is used to treat anemia (iron-deficiency).
- The most common use of iron is in manufacturing of steel, that has various attractive properties and uses.
Health Effects
- Iron can be found in meat, whole meal products, potatoes and vegetables. The human body absorbs iron in animal products faster than iron in plant products. Iron may cause conjunctivitis, choroiditis, and retinitis if it contacts and remains in the tissues. Chronic inhalation of excessive concentrations of iron oxide fumes or dusts may result in development of a benign pneumoconi…
Isotopes of Iron
- Naturally occurring iron consists of four isotopes: 5.85 percent of slightly radioactive 54Fe (half-life >3.1×1022 years), 91.75 percent of stable 56Fe, 2.12 percent of stable 57Fe, and 0.28 percent of stable 58Fe. In addition, it appears that the naturally occurring radioactive isotope 60Fe, with a half-life of 1.5 million years, is now extinct, but it can be produced synthetically. Much of the pas…