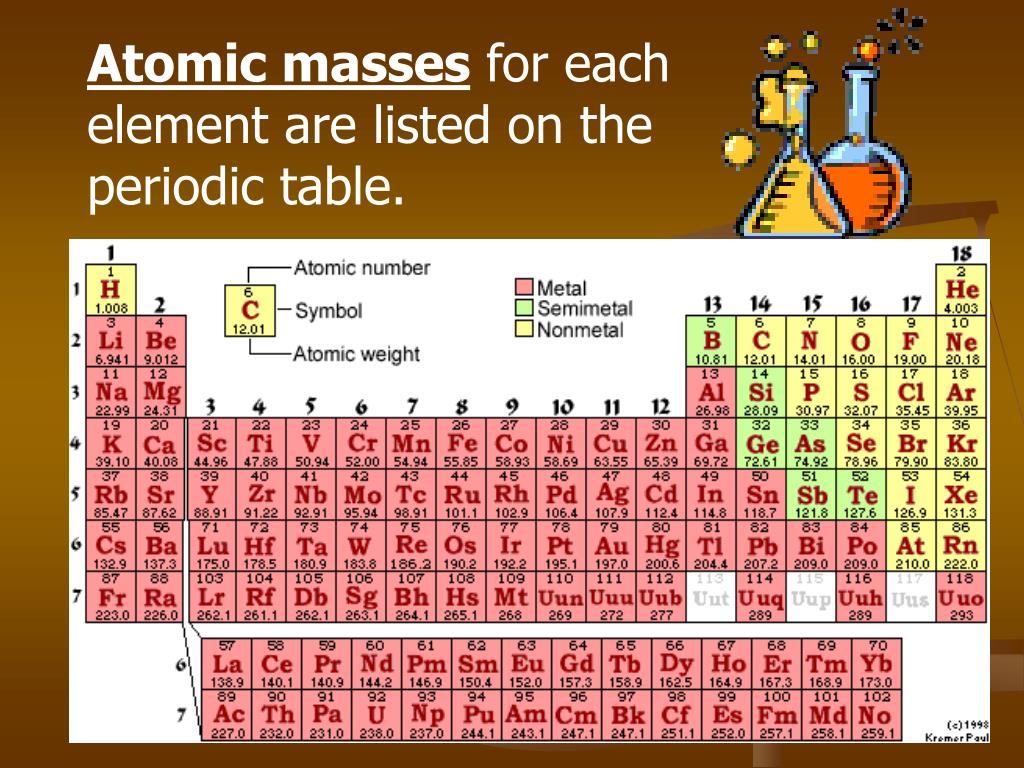
Is Mg on the periodic table a metal or nonmetal?
magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. Its compounds are widely used in construction and medicine, and magnesium is one of the elements essential to all cellular life.
What family is Mg in on the periodic table?
The Alkaline Earth MetalsGroup 2A (or IIA) of the periodic table are the alkaline earth metals: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Is Mg a pure metal?
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which shares many physical and chemical properties with the other five alkaline earth metals (group 2 of the periodic table)....MagnesiumMohs hardness1–2.5Brinell hardness44–260 MPaCAS Number7439-95-4History49 more rows
Is magnesium dust harmful?
* Breathing Magnesium Oxide can irritate the eyes and nose. * Exposure to Magnesium Oxide can cause “metal fume fever.” This is a flu-like illness with symptoms of metallic taste in the mouth, headache, fever and chills, aches, chest tightness and cough.
Why magnesium is a metal?
As magnesium is located in group 2 it is considered as a metal. When metals react with acid it yield salt and hydrogen gas. Accordingly, when magnesium reacts with dilute hydrochloric acid, it yields magnesium chloride (salt) and hydrogen gas as a product.
What are 3 interesting facts about magnesium?
Magnesium is a silvery-white alkaline earth metal. Magnesium is named for the Greek city of Magnesia, a source of calcium oxide, which is called magnesia. Magnesium is the ninth-most abundant element in the universe. Magnesium forms in large stars as a result of the fusion of helium with neon.
Why is the element magnesium so important?
Magnesium is an essential element in both plant and animal life. Chlorophyll is the chemical that allows plants to capture sunlight, and photosynthesis to take place. Chlorophyll is a magnesium-centred porphyrin complex. Without magnesium photosynthesis could not take place, and life as we know it would not exist.
Where is magnesium mined?
Magnesium is also obtained in smaller quantities from the magnesium-bearing minerals dolomite, magnesite, kieserite and brucite. Other magnesium-bearing minerals include carnallite, cordierite, and diopside. Magnesium is mined in China, North Korea, Russia, Austria, Greece and the USA.
What makes magnesium unique?
Cool Facts About Magnesium Magnesium, mg, is the eighth-most abundant element. Magnesium oxide is the second most abundant compound found in the earth's crust. For every cubic kilometer of seawater, there are about 1.3 billion kilograms of magnesium. Magnesium is the most lightweight out of all the metallic elements.
Is it OK to inhale chalk?
Due to the size, chalk dust can be harmful to health when inhaled. Known as respirable dust, chalk dust can be responsible for travelling into the respiratory system and causing effect.
What happens if you smoke magnesium?
Inhalation of magnesium dust or magnesium oxide smoke can produce respiratory irritation with the following potential signs and symptoms: Nasal catarrh. Productive cough. Pneumonitis, including metal fume fever.
Is magnesium metal safe to touch?
Protect from sources of ignition. Avoid contact with skin and eyes. Wash thoroughly before eating or smoking.
What element is in the same family as C?
The carbon family consists of the elements carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). Atoms of elements in this group have four valence electrons. The carbon family is also known as the carbon group, group 14, or the tetrels.
Which element is in row four and group 2?
The given element is calcium.
What compound is magnesium?
Magnesium compounds, primarily magnesium oxide, are used mainly as refractory material in furnace linings for producing iron and steel, nonferrous metals, glass, and cement. Magnesium oxide and other compounds also are used in agricultural, chemical, and construction industries.
Where is magnesium's name from?
Magnesium's name is derived from magnesia, which Davy used in his experiment. Magnesia is the district of Thessaly in Greece where magnesia alba [magnesium carbonate] was found.
Magnesium in Periodic table
Magnesium element is in group 2 and period 3 of the Periodic table. Magnesium is the s-block element and it belongs to alkaline earth metals group.
Properties of Magnesium
The physical and chemical properties of magnesium element are mentioned below.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
Where is magnesium found?
It is found in large deposits in minerals such as magnesite and dolomite. The sea contains trillions of tonnes of magnesium, and this is the source of much of the 850,000 tonnes now produced each year.
How much magnesium is needed for life?
Magnesium is essential to almost all life on Earth - it is at the heart of the chlorophyll molecule, which plants use to convert carbon dioxide into glucose, and then to cellulose, starch, and many other molecules which pass along the food chain. Humans take in around 300 mg of magnesium per day and we need at least 200 mg, but the body has a store of around 25 g of this element in its skeleton so there is rarely a deficiency.
What is magnesium oxide used for?
Magnesium oxide is used to make heat-resistant bricks for fireplaces and furnaces. It is also added to cattle feed and fertilisers. Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine.
Why is magnesium used in car parts?
It improves the mechanical, fabrication and welding characteristics of aluminium when used as an alloying agent. These alloys are useful in aeroplane and car construction. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools.
When was magnesium first discovered?
An impure form of metallic magnesium was first produced in 1792 by Anton Rupprecht who heated magnesia with charcoal. A pure, but tiny, amount of the metal was isolated in 1808 by Humphry Davy by the electrolysis of magnesium oxide. However, it was the French scientist, Antoine-Alexandre-Brutus Bussy who made a sizeable amount of the metal in 1831 by reacting magnesium chloride with potassium, and he then studied its properties.
How are elements organized into blocks?
Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The number of protons in an atom.
What is a vertical column in the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table.
How many protons does magnesium have?
Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The chemical symbol for Magnesium is Mg.
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
How many protons does boron have?
Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure. The chemical symbol for Boron is B.
How is atomic weight determined?
Therefore it is determined by the mass number (number of protons and neutrons).
What is the atomic mass of an atom?
The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
What is the most abundant element in the Earth's crust?
Aluminium is a silvery-white, soft, nonmagnetic, ductile metal in the boron group. By mass, aluminium makes up about 8% of the Earth’s crust; it is the third most abundant element after oxygen and silicon and the most abundant metal in the crust, though it is less common in the mantle below.
What is the temperature of nitrogen?
Liquid nitrogen (made by distilling liquid air) boils at 77.4 kelvins (−195.8°C) and is used as a coolant.
How much magnesium is in the human body?
The average adult human body contains 22 to 26 grams of magnesium, mostly in the skeleton and skeletal muscles. Magnesium deficiency (hypomagnesemia) is common and occurs in 2.5 to 15% of the population. Causes include low calcium consumption, antacid therapy, and loss from the kidneys or gastrointestinal tract.
What is the melting point of magnesium?
Properties: Magnesium has a melting point of 648.8°C, boiling point of 1090°C, specific gravity of 1.738 (20°C), and valence of 2. Magnesium metal is light (one-third lighter than aluminum), silvery-white, and relatively tough. The metal tarnishes slightly in air. Finely divided magnesium ignites upon heating in air, ...
What is magnesium in the universe?
Anne Marie Helmenstine, Ph.D. Updated August 02, 2019. Magnesium is an element that is essential for human nutrition. This alkaline earth metal has atomic number 12 and element symbol Mg. The pure element is a silver-colored metal, but it tarnishes in air to give it a dull appearance. Crystals of pure magnesium metal.
What is magnesium hydroxide?
Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride, and citrate are used in medicine. Organic magnesium compounds have many uses. Magnesium is essential for plant and animal nutrition. Chlorophyll is a magnesium-centered porphyrin.
When was magnesium first discovered?
Discovery: Recognized as an element by Black 1775; Isolated by Sir Humphrey Davy 1808 (England). Magnesium first came into use as magnesium sulfate or Epsom salt. The story goes that in 1618 a farmer in Epsom, England could not get his cattle to drink from a well with bitter-tasting water, yet the water seemed to heal skin conditions.
Where is magnesium found?
Sources: Magnesium is the 8th most abundant element in the earth's crust. While it is not found free it nature, it is available in minerals including magnesite and dolomite. The metal may be obtained by electrolysis of fused magnesium chloride derived from brines and seawater. Atomic Weight: 24.305.
Who was the first person to name magnesium?
Magnesium was originally named 'magnium' by Humphrey Davy after isolating the element from magnesia, known now as magnesium oxide. The 1915 Nobel Prize in Chemistry was awarded to Richard Willstätter for his work with the chlorophyll and identifying magnesium was the central atom in its structure.
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
What is the symbol for electronegativity?
Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. For this purposes, a dimensionless quantity the Pauling scale, symbol χ, is the most commonly used.
How does electronegativity affect a compound?
In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. The most electronegative atom, fluorine, is assigned a value of 4.0, and values range down to cesium and francium which are the least electronegative at 0.7. Electronegativity is related with ionization energy and electron affinity. Electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong attractive force on electrons. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on the negative electrons. Therefore the electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left.
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
What is the most abundant element in the Earth's crust?
Aluminium is a silvery-white, soft, nonmagnetic, ductile metal in the boron group. By mass, aluminium makes up about 8% of the Earth’s crust; it is the third most abundant element after oxygen and silicon and the most abundant metal in the crust, though it is less common in the mantle below.
What is the most common type of boron?
There are over 100 different borate minerals, but the most common are: borax , kernite, ulexite etc. Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Do elements of the same group have the same number of valence electrons?
In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
Do elements in the same period have the same number of energy shells?
The elements in same Periods have the same number of energy shells (or energy orbits).
What exactly is the Atomic radius in Periodic table?
In simple words, the distance from the center of the nucleus to the outermost orbit is known as atomic radius.
How is the atomic radius measured?
How is atomic radius measured? Atomic radius is measured by measuring the distance between the two adjacent atoms, and then dividing that distance by 2, we get the atomic radius. Above image clearly shows you how the atomic radius is measured.
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Is the atomic size of an atom small?
Yes, the atoms are very small in size. You can imagine the relative atomic size in Periodic table from the below image. Must see: Atomic size trend in Periodic table (where you will come to know why and how the size of atoms changes across a period and along a group)
