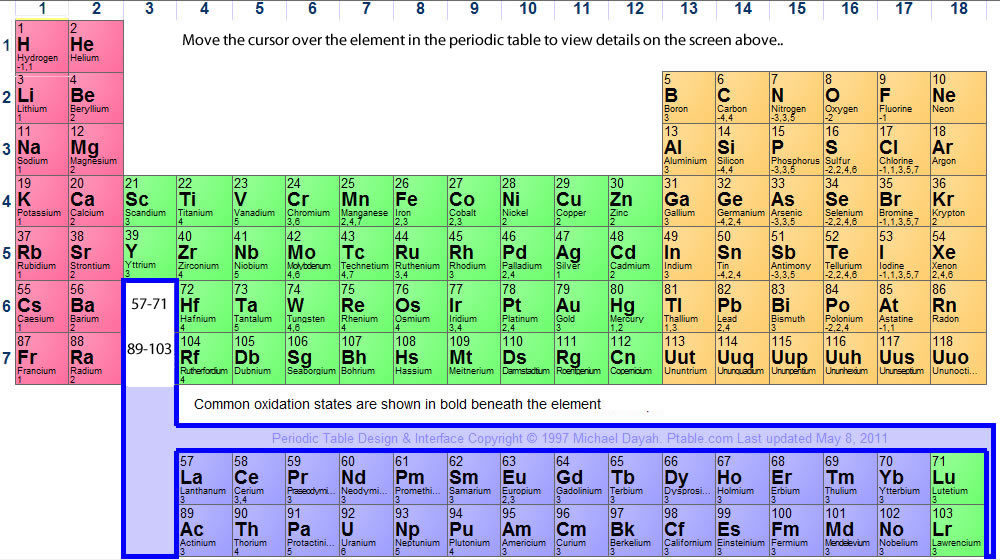
What does 'N' stand for in the periodic table?
N, the chemical symbol for the element nitrogen. N or Asn, the symbol for the common natural ...
What are the non metals on the periodic table?
There are only two exceptions, i.e., two elements in that sequence (between number 5 and number 84) that are not metals: atomic number 32, Germanium (Ge); and atomic number 52, Antinomy (Sb). Everything else to the left of those elements is classified as metal.
What is period 3 on the periodic table?
What is Period 3 on the periodic table? The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block.
What does NE mean on the periodic table?
The name comes from the Greek 'neos', meaning new. A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.
What is the periodic table?
What is the mass number of sodium isotopes?
How many protons and electrons are in hydrogen?
How are atomic nuclei determined?
What are the two forces that make up the nucleus?
What is the charge of an atom?
How to determine the stability of an isotope?
See 4 more
About this website
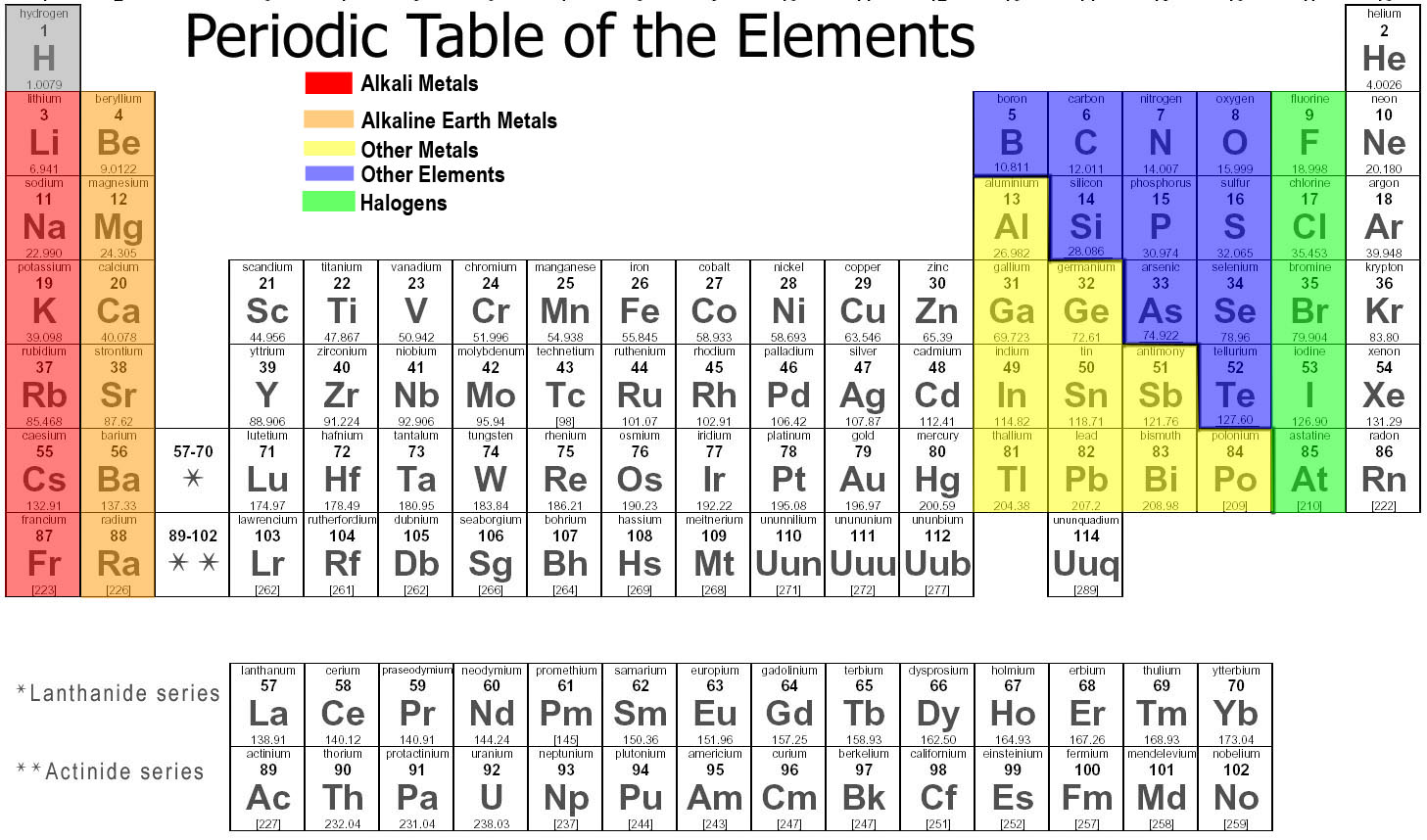
Is Na a metal or nonmetal?
sodium (Na), chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal. Sodium is the most common alkali metal and the sixth most abundant element on Earth, comprising 2.8 percent of Earth's crust.
What does Na stand for in the periodic table?
Sodium (Na – Natrium)
Why sodium is called Na?
Na is the chemical symbol for sodium from the sodium synonym Natrium. Outside of cells, sodium is the most abundant positive ion (cation) in the fluid.
Is pure sodium toxic?
The effects of exposure to pure sodium metal by any route – dermal, inhalation, or ingestion – will most likely result in symptoms similar to those of sodium hydroxide exposure, where the caustic effects of the compound on tissues result in mild to severe burns.
What is the longest element word?
The prize for the longest element name goes to rutherfordium, named after the famous New Zealand physicist Lord Ernest Rutherford who won a Nobel Prize in chemistry. Praseodymium means 'green twin', a name that relates to confusion over the supposed element didymium....science.chemistry.element.periodic table.praseodymium.
What are 5 interesting facts about sodium?
Fun Sodium FactsSodium is a soft, malleable and shiny solid at room temperature.Sodium is less dense than water. ... Sodium is soft enough to cut with a butter knife at room temperature.Sodium metal reacts with water to produce hydrogen gas. ... Sodium burns with a bright yellow light.More items...•
Why is sodium a metal?
Sodium is a metal because it is a good conductor of electricity, malleable and ductile. It can lose electrons easily from the valence shell whereas carbon is a bad conductor of electricity, not lustrous or malleable.
What is sodium used for?
Sodium is used as a heat exchanger in some nuclear reactors, and as a reagent in the chemicals industry. But sodium salts have more uses than the metal itself. The most common compound of sodium is sodium chloride (common salt). It is added to food and used to de-ice roads in winter.
Is salt a metal?
Salts are composed of metal cations and nonmetal anions bonded together through an ionic bond.
Can salt make you vomit?
You may then experience extreme thirst, nausea, dizziness, stomach cramps, vomiting and diarrhea as your system is unable to rid itself of excess sodium.
What would happen if I ate sodium?
But too much sodium in the diet can lead to high blood pressure, heart disease, and stroke. It can also cause calcium losses, some of which may be pulled from bone.
How much sodium is too much a day?
Americans eat on average about 3,400 mg of sodium per day. However, the Dietary Guidelines for Americans recommends adults limit sodium intake to less than 2,300 mg per day—that's equal to about 1 teaspoon of table salt! For children under age 14, recommended limits are even lower.
What is sodium used for?
Sodium is used in the production of titanium, sodamide, sodium cyanide, sodium peroxide, and sodium hydride. Liquid sodium has been used as a coolant for nuclear reactors. Sodium vapor is used in streetlights and produces a brilliant yellow light. Sodium also forms many useful compounds.
What period is sodium Na located?
Solution : From the electronic configuration of sodium (Na = 11 = 2, 8, 1), we find that there is one electron in its outermost shell (third shell). Therefore, sodium is an element of group 1 and period 3.
What two elements make a laser pen?
A laser pointer or laser pen is a small handheld device with a power source (usually a battery) and a laser diode emitting a very narrow coherent low-powered laser beam of visible light, intended to be used to highlight something of interest by illuminating it with a small bright spot of colored light.
What is sodium protons neutrons and electrons?
An atom of sodium has 11 protons, 12 electrons, and 11 neutrons.
Chemical Symbol for Sodium - Na - Periodic Table
Chemical Symbol for Sodium. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure.The chemical symbol for Sodium is Na. Sodium is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table, because it has a single electron in its outer shell that it readily donates ...
Sodium - Atomic Mass - Atomic Weight - Na - Periodic Table
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
How many electrons does Na have?
This electron arrangement indicates that the outermost orbit of Sodium (Na) has 1 electron.
What group is sodium in?
Sodium element is in group 1 and period 3 of the Periodic table. Sodium is the s-block element and it belongs to alkali metals group.
When sodium metal is heated with a flame, what happens to its electrons?
When sodium metal is heated with a flame, its outermost electron gets excited onto a higher energy level.
What color is sodium metal?
Sodium metal is silver white in color when it is freshly cut, but it suddenly forms an oxide layer if kept open in air.
Which element has less intermolecular force of attraction?
In short sodium element has less intermolecular force of attraction.
Which element has the largest atomic radius?
Sodium element is in group 1, and group 1 elements have the larger atomic radius compared to elements of other groups.
Which block will the elements lie in?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
Where does the name Na come from?
The name derives from the English soda and Latin sodanum for "headache remedy". The symbol Na derives from the Latin natrium for "natron" (soda in English). Sodium was discovered in 1807 by the English chemist Humphry Davy from electrolysis of caustic soda (NaOH).
What is sodium used for?
Sodium is used in the production of titanium, sodamide, sodium cyanide, sodium peroxide, and sodium hydride. Liquid sodium has been used as a coolant for nuclear reactors. Sodium vapor is used in streetlights and produces a brilliant yellow light. Sodium also forms many useful compounds.
How is sodium obtained?
It is now obtained commercially by the electrolysis of absolutely dry fused sodium chloride.
What is the most common compound in the world?
The most common compound is sodium chloride (table salt), but it occurs in many other minerals, such as soda niter, cryolite, amphibole, zeolite, etc. Los Alamos National Laboratory, U.S. Department of Energy. Sodium compounds are important to the paper, glass, soap, textile, petroleum, chemical, and metal industries.
Why is sodium important?
Metallic sodium is vital in the manufacture of esters and in the preparation of organic compounds. The metal may be used to improve the structure of certain alloys, descale metal, and purify molten metals. An alloy of sodium with potassium, NaK, is an important heat transfer agent.
Can sodium metal be handled in an inert atmosphere?
Sodium metal should be handled with great care. It cannot be maintained in an inert atmosphere and contact with water and other substances with which sodium reacts should be avoided.
What is the atomic mass of sodium?
Sodium has an atomic number of 1 and atomic mass of 22.98. It is placed in group 1 of periodic table as it has a single electron in its outer most shell that it readily donates, creating a positively charged ion, the Na+ cation. At room temperature Sodium is soft, silvery-white metal which can be easily cut with a knife. It is highly reactive. In air it reacts with oxygen forming grayish white sodium oxide, so it is stored in an inert liquid such as kerosene or in nitrogen gas as it does not react with nitrogen. It is lighter than water. It is a good conductor of heat and electricity and exhibits the photoelectric effect i.e. the emission of electrons when exposed to light. Sodium and its compounds give yellow color to the flame which is the basic analytical test for sodium. It burns at a temperature more than 800 °C (1,500 °F) [5].
What is sodium made of?
Sodium in the form of salt (sodium chloride, NaCl) and soda (sodium carbonate, Na2CO3) have been known since prehistoric times [1] The name of element originated from Arabic word ‘suda’ which means headache, as the association of sodium with headache was known in early times. The metal was first isolated in 1807, through the electrolysis of sodium hydroxide by Sir Humphry Davy. In 1809, Ludwig Wilhelm, a German physicist and chemist, proposed the name Natronium for it. Its chemical abbreviation ‘Na’ was published in the system of atomic symbols in 1814 which is derived from its Latin name ‘natrium’ [2].
How much sodium is needed for a healthy body?
Around 500mg of sodium is the minimum physiological requirement per day of human body. Sodium Chloride is daily used as seasoning and preservative in diet. In plants, sodium is a micronutrient which helps in metabolism. It is involved in synthesis of chlorophyll, opening and closing of stomata, and helps in uptake of water.
What is the reaction of sodium and carbon?
It is relatively less reactive with carbon, but at 625°C it reacts with carbon monoxide forming sodium carbide and sodium carbonate. With liquid ammonia sodium reacts to give blue colored solutions forming sodamide, but the reaction is quite slow. Organic acids also react with sodium to form sodium salts.
What is the importance of sodium in the cell?
Significance and Uses. Sodium is one of the most essential elements for life, as sodium and potassium keep a definite balance within the cell and are involved in maintaining an electrolyte balance across the cell membranes [6]. It helps in the regulation of blood pressure, blood volume, osmotic equilibrium and pH.
What are the characteristics of sodium hydroxide?
Chemical Characteristics. It is highly reactive in air and forms sodium hydroxide (NaOH), which is a strong base on reaction with water. In presence of air, sodium hydroxide film absorbs carbon dioxide, and lead to the formation of sodium bicarbonate. Sodium reacts with halogens under certain conditions to produce light.
How many isotopes of sodium are there?
Isotopes of Sodium. Twenty isotopes of sodium are identified, but only one, sodium-23 is stable. Two radioactive, cosmogenic isotopes which are the byproduct of cosmic ray spallation are known, Na-22 which has a half-life of 2.6 years and Na-24 with a half-life of 15 hours.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the symbol for sodium?
The element symbol, Na, was shortened from the Latin name 'Natrium.'. Swedish chemist Berzelius was the first to use the symbol Na for sodium in his early periodic table. History: Sodium does not usually appear in nature on its own, but its compounds have been used by people for centuries.
What is sodium metal used for?
Sodium metal may be used to improve the structure of some alloys, to descale metal, and to purify molten metals. Sodium, as well as NaK, an alloy of sodium with potassium, are important heat transfer agents.
What is sodium chloride used for?
Sodium chloride is important for animal nutrition. Sodium compounds are used in the glass, soap, paper, textile, chemical, petroleum, and metal industries. Metallic sodium is used in manufacturing of sodium peroxide, sodium cyanide, sodamide, and sodium hydride. Sodium is used in preparing tetraethyl lead. It is used in the reduction of organic esters and preparation of organic compounds. Sodium metal may be used to improve the structure of some alloys, to descale metal, and to purify molten metals. Sodium, as well as NaK, an alloy of sodium with potassium, are important heat transfer agents.
What is the 6th most abundant element in the Earth's crust?
Sodium is the 6th most abundant element in the Earth's crust, making up approximately 2.6% of the earth, air, and oceans.
Which country produces the most sodium?
The top three countries that produce sodium are China , United States, and India. Sodium metal is mass produced by electrolysis of sodium chloride. The D lines of sodium's spectrum account for the dominant yellow color of the un. Sodium is the most abundant alkali metal.
Does sodium float in water?
Sodium floats on water, which decomposes it to evolve hydrogen and form the hydroxide. Sodium may ignite spontaneously on water. It does not usually ignite in air at temperatures below 115°C
What is the periodic table?
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers , electron configurations, and chemical properties. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements.
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
What is the charge of an atom?
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
What are the two forces that make up the nucleus?
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
How to determine the stability of an isotope?
To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue. Only two stable nuclides have fewer neutrons than protons: hydrogen-1 and helium-3.
How are the chemical properties of a solid, liquid, gas, and plasma determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
What is the periodic table?
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements.
What is the mass number of sodium isotopes?
Mass numbers of typical isotopes of Sodium are 23.
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
What are the two forces that make up the nucleus?
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
What is the charge of an atom?
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
How to determine the stability of an isotope?
To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue. Only two stable nuclides have fewer neutrons than protons: hydrogen-1 and helium-3.

Occurrence
Physical Characteristics
- Sodium has an atomic number of 1 and atomic mass of 22.98. It is placed in group 1 of periodic table as it has a single electron in its outer most shell that it readily donates, creating a positively charged ion, the Na+ cation. At room temperature Sodium is soft, silvery-white metal which can be easily cut with a knife. It is highly reactive. In a...
Chemical Characteristics
- It is highly reactive in air and forms sodium hydroxide (NaOH), which is a strong base on reaction with water. In presence of air, sodium hydroxide film absorbs carbon dioxide, and lead to the formation of sodium bicarbonate. Sodium reacts with halogens under certain conditions to produce light. Halogen acids e.g. hydrofluoric and hydrochloric acid react vigorously with sodiu…
Significance and Uses
- Sodium is one of the most essential elements for life, as sodium and potassium keep a definite balance within the cell and are involved in maintaining an electrolyte balance across the cell membran...
- It helps in the regulation of blood pressure, blood volume, osmotic equilibrium and pH. Around 500mg of sodium is the minimum physiological requirement per day of human body.
- Sodium is one of the most essential elements for life, as sodium and potassium keep a definite balance within the cell and are involved in maintaining an electrolyte balance across the cell membran...
- It helps in the regulation of blood pressure, blood volume, osmotic equilibrium and pH. Around 500mg of sodium is the minimum physiological requirement per day of human body.
- Sodium Chloride is daily used as seasoning and preservative in diet. In plants, sodium is a micronutrient which helps in metabolism. It is involved in synthesis of chlorophyll, opening and closing...
- Significant amounts of sodium are used to produce synthetic detergents, dyes, intermediates of dye and perfumes.
Health Hazards
- According to The US Institute of Medicine, the Tolerable Upper Intake Level for sodium is 2.3 grams per day. A decrease in sodium intake would lead to fewer cases of hypertension . Hypertension causes almost 7.6 million premature deaths worldwide every year. The American Heart Association recommends not more than 1.5 g of sodium per day .
Isotopes of Sodium
- Twenty isotopes of sodium are identified, but only one, sodium-23 is stable. Two radioactive, cosmogenic isotopes which are the byproduct of cosmic ray spallation are known, Na-22 which has a half-life of 2.6 years and Na-24 with a half-life of 15 hours. All other isotopes have a half-life of less than one minute [10, 11].