
What is 46 on the periodic table?
Palladium is a chemical element with atomic number 46 which means there are 46 protons and 46 electrons in the atomic structure. The chemical symbol for Palladium is Pd. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs).
What is the formula for phosphorus?
Phosphorus has an oxidation number of −3 in phosphine. Phosphine is produced by hydrolysis of calcium phosphide, Ca 3 P 2. Unlike ammonia, phosphine is oxidised by air. Phosphine is also far less basic than ammonia. Other phosphines are known which contain chains of up to nine phosphorus atoms and have the formula P n H n+2.
What are facts about phosphorus?
Facts About Phosphorus. Phosphorus is in Period 3 and Group 15 of the periodic table. It has 15 protons, so it has the atomic number 15. This also means it is the 15th element on the periodic table.
What is the classification of phosphorus?
Phosphorus is a chemical element with symbol P and atomic number 15. Classified as a nonmetal, ...
See more
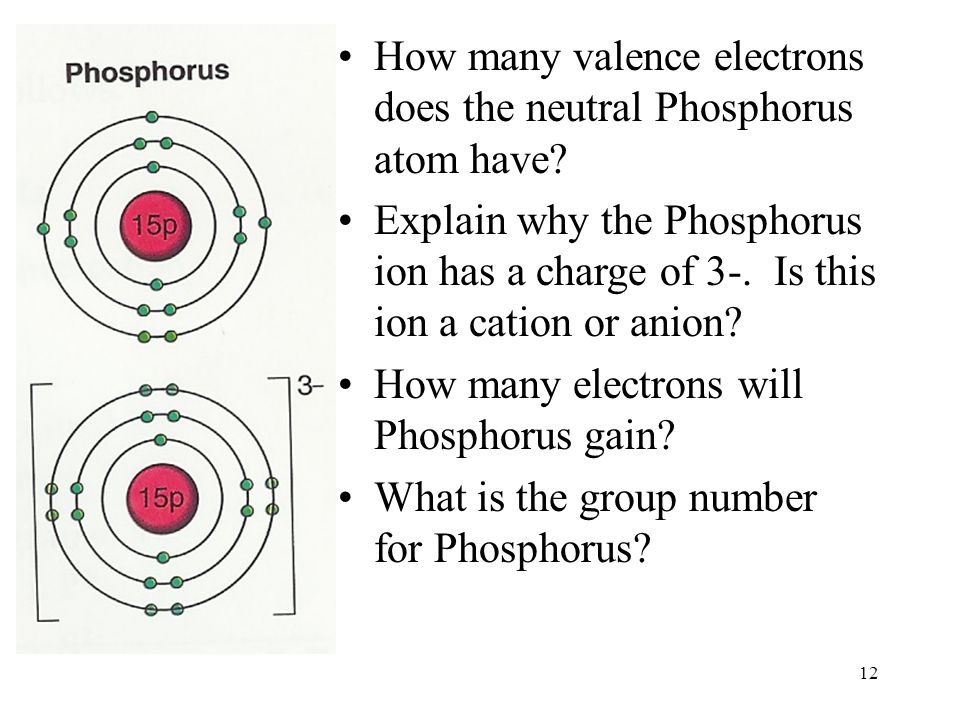
Why is phosphorus A element?
Phosphorus is an element that one will under no circumstances find freely in our environment. It is extremely reactive. Phosphorus is a chemical element with an atomic number of 15, which means that the atomic structure includes 15 protons and 15 electrons. For Phosphorus, the chemical symbol is P.
What are the 3 types of phosphorus?
The important allotropic forms of phosphorus are white phosphorus, black phosphorus and red phosphorus.
What is phosphorus mainly used for?
The main function of phosphorus is in the formation of bones and teeth. It plays an important role in how the body uses carbohydrates and fats. It is also needed for the body to make protein for the growth, maintenance, and repair of cells and tissues.
What are the 4 common forms of phosphorus?
Phosphorus has different colours -- white phosphorus, red phosphorus, violet phosphorus and black phosphorus -- depending on the arrangements of the bonds it forms.
Is phosphorus poisonous to humans?
Ingestion of elemental white or yellow phosphorus typically causes severe vomiting and diarrhea, which are both described as “smoking,” “luminescent,” and having a garlic-like odor. Other signs and symptoms of severe poisoning might include dysrhythmias, coma, hypotension, and death.
Is phosphorus a metal or nonmetal?
non-metalPhosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. This element exists in several forms, of which white and red are the best known.
What foods contain phosphorus?
Phosphorus can be found in foods (organic phosphorus) and is naturally found in protein-rich foods such as meats, poultry, fish, nuts, beans and dairy products. Phosphorus found in animal foods is absorbed more easily than phosphorus found in plant foods.
What will happen if phosphorus runs out?
Phosphorus is an essential nutrient for all forms of life. It is a key element in our DNA and all living organisms require daily phosphorus intake to produce energy. It cannot be replaced and there is no synthetic substitute: without phosphorus, there is no life.
Where is phosphorus most commonly found?
Phosphorus is most commonly found in rock formations and ocean sediments as phosphate salts. Phosphate salts that are released from rocks through weathering usually dissolve in soil water and will be absorbed by plants.
Why is phosphorus called the devil's element?
White phosphorus has been called the “devil's element” because it glows green in the dark and is pyrophoric. Because of its instability, white phosphorus is typically stored under water, in which it is barely soluble.
Where can phosphorus be found in a home?
Go take a look in your refrigerator or cupboards. Maybe your mom keeps baking powder in there for when she needs to do some cooking. When you see that white powder you'll know that scientists used phosphorus to make it.
What happens when phosphorus reacts with water?
In water, white phosphorus reacts with oxygen within hours or days. In water with low oxygen, white phosphorus may degrade to a highly toxic compound called phosphine, which eventually evaporates to the air and is changed to less harmful chemicals.
What is the most common form of phosphorus?
white phosphorusThe most common forms are white phosphorus, made up of phosphorus atoms arranged like a tetrahedron (a four-sided pyramid), and red phosphorus, a solid but non-crystalline form of the element.
What's the difference between phosphorus and red phosphorus?
Summary – Red vs White Phosphorus The key difference between red and white phosphorus is that red phosphorus appears as dark red colored crystals whereas white phosphorus exists as a translucent waxy solid that quickly becomes yellow when exposed to light.
What happens when your phosphorus is low?
Normal blood phosphorous levels are between 2.5 to 4.5 mg/dL. Hypophosphatemia is a condition in which your blood has a low level of phosphorous. Low levels can cause a host of health challenges, including muscle weakness, respiratory or heart failure, seizures, or comas.
What food is high in phosphorus?
High-phosphorus foods to avoid or limit:Dairy foods.Beans.Lentils.Nuts.Bran cereals.Oatmeal.Colas and other drinks with phosphate additives.Some bottled ice tea.
What does phosphorus do to the human body?
Phosphorus compounds, called phosphates, play vital roles in the human body. These compounds are a part of DNA molecules, and are vital to the dist...
What is phosphorus element used for?
Phosphorus is often used to produce agricultural fertilizers, detergents, and matchsticks. It is also used in the production of certain types of ra...
Why is phosphorus called the devil's element?
Historically, phosphorus was sometimes referred to as the 'devil's element.' This was due to its ability to spontaneously burst into flames and glo...
What is phosphorus on the periodic table?
Phosphorus is a chemical element on the periodic table. It is found in group 15 and period 5 of the table.
Can phosphorus kill you?
White phosphorus (an allotrope of phosphorus) is considered to be highly toxic to humans. Exposure to this substance can result in health hazards a...
What happens when phosphorus is too high?
An excess of phosphate in the human body can cause a variety of health issues. These include kidney damage and osteoporosis. This is why it is impo...
Where did phosphorus come from?
So where did it all begin? Phosphorus was first made by Hennig Brandt in Hamburg in Germany in 1669. When he evaporated urine and heated the residue until it was red hot. Glowing phosphorus vapour came off and he condensed it under water. And for more than 100 years most phosphorus was made this way. This was until people realised that bone was a great source of phosphorus. Bone can be dissolved in sulfuric acid to form phosphoric acid, which is then heated with charcoal to form white phosphorus.
When was phosphorus first made?
Elements and Periodic Table History. Phosphorus was first made by Hennig Brandt at Hamburg in 1669 when he evaporated urine and heated the residue until it was red hot, whereupon phosphorus vapour distilled which he collected by condensing it in water.
Why is phosphate important for life?
Phosphorus is essential to all living things. It forms the sugar-phosphate backbone of DNA and RNA. It is important for energy transfer in cells as part of ATP (adenosine triphosphate), and is found in many other biologically important molecules. We take in about 1 gram of phosphate a day, and store about 750 grams in our bodies, since our bones and teeth are mainly calcium phosphate. Over-use of phosphates from fertilisers and detergents can cause them to pollute rivers and lakes causing algae to grow rapidly. The algae block out light stopping further photosynthesis. Oxygen dissolved in the water soon gets used up and the lake dies.
What is the most common use of phosphorus compounds?
By far the largest use of phosphorus compounds is for fertilisers. Ammonium phosphate is made from phosphate ores. The ores are first converted into phosphoric acids before being made into ammonium phosphate.
Why are phosphates being phased out?
This is because they can lead to high phosphate levels in natural water supplies causing unwanted algae to grow. Phosphates are also used in the production of special glasses and fine chinaware. Biological role.
What is the purpose of red phosphorus?
White phosphorus is used in flares and incendiary devices. Red phosphorus is in the material stuck on the side of matchboxes, used to strike safety matches against to light them. By far the largest use of phosphorus compounds is for fertilisers.
Where is phosphate found?
Phosphorus is not found uncombined in nature, but is widely found in compounds in minerals. An important source is phosphate rock, which contains the apatite minerals and is found in large quantities in the USA and elsewhere.
Where is phosphorus found in the periodic table?
Phosphorus is a chemical element on the periodic table. It is found in group 15 and period 5 of the table.
What is Phosphorus?
What is phosphorus? Phosphorus is one of 118 chemical elements found on the periodic table. A chemical element is a substance that will contain only one type of atom in a pure sample. The atoms of all elements have distinct physical and chemical properties and atomic compositions (e.g., the number of protons or electrons in each atom).
What element has a red circle on the periodic table?
The periodic table of elements. Phosphorus has been highlighted with a red circle.
What is the atomic mass of phosphorus?
The atomic mass of phosphorus (30.974) represents the average mass of all naturally occurring phosphorus atoms on Earth in atomic mass units (or daltons, D). It also allows the calculation of the number of neutrons in a phosphorus atom, which is equal to 30.974-15 = 16 neutrons.
What is the name of the phosphorus that reacts with oxygen in the air?
White phosphorus readily catches fire and reacts with oxygen in the air to form P4O10. Red phosphorus reacts with oxygen only at high temperatures (565 K) and forms P4O10. Black phosphorus does not react with oxygen.
What are the properties of phosphorus?
Phosphorus properties can be divided into atomic properties, physical properties, and chemical properties. Atomic properties include the number of protons, electrons, neutrons, and isotopes. Physical properties include characteristics such as color, melting point, boiling point, and hardness. Chemical properties include reactivity and common compounds. The major difference between physical and chemical properties is that the physical properties can be measured without changing the chemical identity, while chemical properties cannot.
Which phosphorus is the least reactive?
As previously discussed, the reactivity of phosphorus varies depending on the allotrope - with White phosphorus being the most reactive and Black phosphorus being the least reactive. Some reaction examples include:
Phosphorus in Periodic table
Phosphorus element is in group 15 and period 3 of the Periodic table. Phosphorus is the p-block element and it belongs to Pnictogens group.
Properties of Phosphorus
The physical and chemical properties of phosphorus element are mentioned below.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
What is the color of phosphorus?
White phosphorus is very reactive element. In the presence of oxygen, white phosphorus emits a light green glow, termed as chemiluminescence (glow caused by cold chemical reaction) [5]. The most abundant compounds of phosphorus contain the tetrahedral anion of phosphate (PO 43- ). There is a vast variety of phosphorus compounds including oxoacids (phosphoric acid), sulfides, nitrides (phosphorus nitride halogens (F2PN, Cl2PN), and phosphides (reaction of metals with red phosphorus). Phosphine (PH3) is a toxic compound with pungent smell, is structural analogue of ammonia. Diphosphine (P2H4), is an analogue of hydrazine and is highly flammable.
Where did the name phosphorus come from?
The name phosphorus also has an interesting origin, as Phosphorus is the name of planet Venus in Ancient Greece language and it means “carrier of light” or “light-bringer”. Commercial scale production of phosphorus was started by Brand and later many scientists, including Robert Boyle, used the same method of phosphorus production developed by ...
What are the physical characteristics of phosphorus?
Physical Characteristics. White phosphorus is yellowish white solid, that has a waxy texture. Phosphorus undergoes spontaneous ignition in air and forms pentoxide (P 4 O 10 ). There are two allotropic forms of phosphorus, red and black, that differ in physical and chemical properties. Red phosphorus (which is formed by heating ...
What is the role of phosphorus in the cell membrane?
Phosphorylation, that is the process of adding phosphate to various biological molecules, is an important regulatory mechanism in living organisms. Lipids in combination with phosphorus (phospholipids) are the primary building blocks of cell membrane. Phosphorus is present in the building blocks of RNA and DNA.
How much phosphorus is in the human body?
About 0.7 kg of phosphorus is present in an average adult human being, mostly in teeth and bones and in soft tissues of the body. deficiency of phosphate in the body can lead to various physiological effects, including tissue weakness, neurological defects and lack of ATP.
What is phosphorus used for?
Phosphorus is used in making detergents, that functions to remove water hardness and improve the efficiency of detergent. It is used in synthesis of nerve agents. It is used as active ingredient in various pesticides. Phosphoric acid is widely used in manufacturing of soft drinks, baking powder.
What is the role of phosphate in the cell?
Phosphorus in the form of phosphate is a vital compound for all living systems. The energy currency of cell, ATP (adenosine triphosphate) that regulates every process in the living cell, uses phosphate. Phosphorylation, that is the process of adding phosphate to various biological molecules, is an important regulatory mechanism in living organisms. Lipids in combination with phosphorus (phospholipids) are the primary building blocks of cell membrane. Phosphorus is present in the building blocks of RNA and DNA. A balanced diet includes a definitive everyday intake of phosphorus. About 0.7 kg of phosphorus is present in an average adult human being, mostly in teeth and bones and in soft tissues of the body. deficiency of phosphate in the body can lead to various physiological effects, including tissue weakness, neurological defects and lack of ATP. Increased intake of phosphate can cause hardening of tissues and organs and diarrhea.
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
How many electrons does neon have?
Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. The chemical symbol for Neon is Ne.
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N.
What is the atomic number of titanium?
Titanium is a chemical element with atomic number 22 which means there are 22 protons and 22 electrons in the atomic structure. The chemical symbol for Titanium is Ti. Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. Titanium can be used in surface condensers. These condensers use tubes that are usually made of stainless steel, copper alloys, or titanium depending on several selection criteria (such as thermal conductivity or corrosion resistance). Titanium condenser tubes are usually the best technical choice, however titanium is very expensive material.
What is the most common type of boron?
There are over 100 different borate minerals, but the most common are: borax , kernite, ulexite etc. Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B.
Which element has the same electron configuration in the outer electron shell?
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
What is the function of phosphorus?
The phosphorus's function in the human body is to allow formation and healthy state of bones and teeth. This occurs as calcium and phosphate both combine in the bones and in teeth to form calcium phosphate salts. These salts are very important for hardness and structural integrity of teeth.
Where is phosphorus found in the body?
It plays many crucial roles in function and survival. Phosphorus is found in the body in the bones and teeth. Similar to calcium, phosphorus is important to build strong bony structure. The human body has 206 bones in total. Thus, phosphorus is the second most abundant mineral within the human body.
What Does Phosphorus Do for the Body?
The term phosphorus is derived from Greek words that mean light and carry. Thus, phosphorus means light carrier. It was named after another name for the planet Venus. The word phosphorous is different from phosphorus. The term phosphorous is an adjective (whereas phosphorus is a noun) which is used to describe compounds that it reacts it. For example, a compound of sulfur would be called sulfuric and sulfurous depending on ionic state of sulfur in the compound. Similarly, phosphorous and phosphoric are terms used to describe compounds of phosphorus with other elements. Phosphorous is phosphorus in P3+ ionic state, whereas phosphoric is phosphorus in P5+ ionic state.
What is the cause of low phosphorus levels?
Hypophosphotemia is a genetic disease that results when too much phosphorus is removed or excreted from the body. Muscle weakness is a symptom of low phosphorus. The normal range is 2.5-4.5 mg/dL of blood. Any levels below 2.5 mg/dL indicate low phosphorus levels. This typically results from eating a poor diet or presence of eating disorders. Issue with storage of phosphorus in the body can also cause low levels as well as presence of low levels of Vitamin D. Vitamin D is crucial for proper absorption of calcium and phosphorus in the human body. Two medical conditions that can arise from this are rickets and osteomalacia.
What is the normal range of phosphorus in the blood?
The normal range of phosphorus in the blood is 2.5-4.5 mg/dL of blood. Any levels that fall below 2.5 mg/dL indicate low phosphorus levels or hypophosphatemia.
How does phosphorus help the body?
Phosphorus helps the human body by balancing with calcium. It also helps to form calcium phosphate salts to provide structure and strength to bones and teeth.
Why is phosphorus important?
These form as phosphorus combines with fats or lipids in the body. Phosphorus is also used to create protein for maintenance of cells, tissues, and organs in the human body. Phosphorus also helps create framework or backbone of DNA and RNA strands. Finally, ATP or adenosine triphosphate is the primary form of energy for human body. Phosphorus plays an important role in formation of this molecule so that humans can create energy and use it to survive, grow, and thrive.
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
When was the periodic table first published?
Periodic table at the Chemical Auditorium of the Gdańsk University of Technology, 1904. The first version of Mendeleev's periodic table, 1 March 1869 (N.S.): An attempt at a system of elements based on their atomic weights and chemical similarities.
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What is the electron configuration of a neutral atom?
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered 1, 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram. The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
How many electrons are in neon?
The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to ...
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
What is phosphorus in the periodic table?
phosphorus (P), nonmetallic chemical element of the nitrogen family (Group 15 [Va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid that glows in the dark.
What is the source of phosphorus?
About 550 different minerals have been found to contain phosphorus, but, of these, the principal source of phosphorus is the apatite series in which calcium ions exist along with phosphate ions and variable amounts of fluoride, chloride, or hydroxide ions, according to the formula [Ca 10 (PO 4) 6 (F, Cl, or OH) 2 ].
What is the most common commercial source of phosphorus?
The chief commercial source is phosphorite, or phosphate rock, an impure massive form of carbonate-bearing apatite. Estimates of the total phosphate rock in Earth’s crust average about 65,000,000,000 tons, of which Morocco and Western Sahara contain about 80 percent. This estimate includes only ore that is sufficiently rich in phosphate for conversion to useful products by present methods. Vast quantities of material lower in phosphorus content also exist.
What is phosphorus used for?
Pyrotechnic applications of the element include tracers, incendiaries, fireworks, and matches. Some is used as an alloying agent, some is used to kill rodents, and the rest is employed in chemical synthesis.
How many atoms of silicon are in phosphorus?
Its cosmic abundance is about one atom per 100 atoms of silicon, the standard. Its high chemical reactivity assures that it does not occur in the free state (except in a few meteorites ). Phosphorus always occurs as the phosphate ion. The principal combined forms in nature are the phosphate salts.
How long does a mass 32 radioactive isotope last?
All of these are radioactive with relatively short half-lives. The isotope of mass 32 has a half-life of 14.268 days and has proven extremely useful in tracer studies involving the absorption and movement of phosphorus in living organisms.
Where is fluoroapatite found?
Very large sedimentary deposits of fluoroapatite are found in many parts of Earth. The phosphate of bone and tooth enamel is hydroxyapatite. (The principle of lessening tooth decay by fluoridation depends upon the conversion of hydroxyapatite to the harder, more decay-resistant, fluoroapatite.)

Occurrence
Physical Characteristics
- White phosphorus is yellowish white solid, that has a waxy texture. Phosphorus undergoes spontaneous ignition in air and forms pentoxide (P4O10). There are two allotropic forms of phosphorus, red and black, that differ in physical and chemical properties. Red phosphorus (which is formed by heating of white phosphorus at high temperature) ignites on friction. White phosph…
Chemical Characteristics
- White phosphorus is very reactive element. In the presence of oxygen, white phosphorus emits a light green glow, termed as chemiluminescence (glow caused by cold chemical reaction) . The most abundant compounds of phosphorus contain the tetrahedral anion of phosphate (PO43-). There is a vast variety of phosphorus compounds including oxoacids (phosphoric acid), sulfides…
Uses and Significance
- The largest use of phosphorus is in the production of fertilizers. It is an essential nutrient for the growth of plants.
- Phosphorus is used in making of safety matches, and various ammunitions, such as incendiary shells and hand grenades etc.
- Phosphorus is used in the manufacturing of bronze and steel.
- The largest use of phosphorus is in the production of fertilizers. It is an essential nutrient for the growth of plants.
- Phosphorus is used in making of safety matches, and various ammunitions, such as incendiary shells and hand grenades etc.
- Phosphorus is used in the manufacturing of bronze and steel.
- It is used in making LEDs (light-emitting diodes).
Health Hazards
- Phosphorus in the form of phosphate is a vital compound for all living systems. The energy currency of cell, ATP (adenosine triphosphate) that regulates every process in the living cell, uses phosphate. Phosphorylation, that is the process of adding phosphate to various biological molecules, is an important regulatory mechanism in living organisms. Lipids in combination wit…
Isotopes of Phosphorus
- There are twenty-three isotopes of phosphorus, that range in atomic numbers from 26 to 43. Natural phosphorus constitutes only one stable isotope, phosphorus-31 .