What is the periodic symbol for salt?
NaSodiumAtomic Number:11227 pm (Van der Waals)Atomic Symbol:Na97.8 °CAtomic Weight:22.99883 °CElectron Configuration:[Ne]3s11
Where is salt on the periodic table?
Sodium in the Periodic Table. Sodium, atomic symbol Na, has an atomic number of eleven, and lies in group 1 on the periodic table, below lithium and to the left of magnesium. This element is in the alkali metals family, and it has only one valence electron.
Is salt a periodic element?
sodium (Na), chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal....Read a brief summary of this topic.atomic number11atomic weight22.9898melting point97.81 °C (208 °F)boiling point882.9 °C (1,621 °F)specific gravity0.971 (20 °C)2 more rows
What is the table name for salt?
Sodium chlorideSodium chloride is often referred to as table salt, common salt, or just simply salt.
Are salts metals?
Salts are composed of metal cations and nonmetal anions bonded together through an ionic bond.
What is the formula of salt?
NaClSodium chloride / FormulaTo most people, salt refers to table salt, which is sodium chloride. Sodium chloride forms from the ionic bonding of sodium ions and chloride ions. There is one sodium cation (Na+) for every chloride anion (Cl–), so the chemical formula is NaCl (Fig.
What makes a salt a salt?
A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. The reaction between an acid and a base is called a neutralization reaction. The term salt is also used to refer specifically to common table salt, or sodium chloride.
Why sodium is called Na?
Na is the chemical symbol for sodium from the sodium synonym Natrium. Outside of cells, sodium is the most abundant positive ion (cation) in the fluid.
Why sodium is a metal?
Sodium is a metal because it is a good conductor of electricity, malleable and ductile. It can lose electrons easily from the valence shell whereas carbon is a bad conductor of electricity, not lustrous or malleable.
Is all salt NaCl?
All salt is sodium chloride (NaCl), and it all comes from seawater — even table salt.
Why is it called table salt?
Table salt is the granulated white salt seen in most saltshakers. Table salt is typically mined from underground deposits. It's processed to remove other minerals. Table salt is commonly fortified with iodine, which is important for thyroid health.
Is table salt a metal?
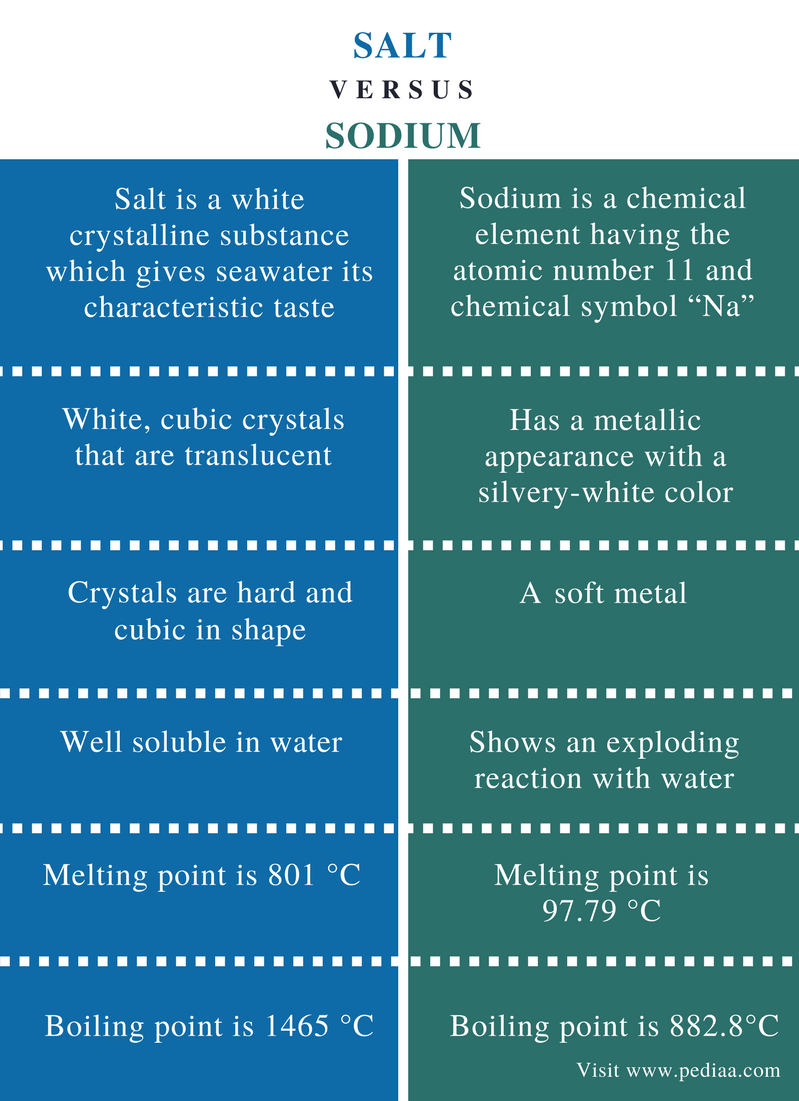
It is mainly composed of sodium chloride. Sodium is a chemical element in group 1 of the periodic table. It is a metal. The main difference between salt and sodium is that salt is a white crystalline compound that is composed of sodium chloride whereas sodium is a metallic element.
Is salt a mineral?
Salt, also known as table salt, or rock salt, is a crystalline mineral that is composed primarily of sodium chloride (NaCl), a chemical compound. Salt for human consumption is produced in different forms: unrefined salt (such as sea salt), refined salt (table salt), and iodized salt.
Is water a salt?
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride). On the United States Geological Survey (USGS) salinity scale, saline water is saltier than brackish water, but less salty than brine.
Is salt water an element compound or mixture?
homogeneous mixtureSaltwater acts as if it were a single substance even though it contains two substances—salt and water. Saltwater is a homogeneous mixture, or a solution.
Which of the following is a salt?
Complete answer: NaCl is a salt, it is a common salt which is not formed from living matter. It is made when sodium atoms and chlorine atoms combine together.
What is a salt made of?
Salt is made of metal cations and nonmetal anions. The cations could be the alkali metal elements from groups 1 and 2, transition metals from group...
What is an example of a salt in chemistry?
Sodium chloride NaCl, table salt, is an example. It is formed through the neutralization reaction of hydrochloric acid and sodium hydroxide. It is...
Is salt a compound or mixture?
Salts are ionic compounds. They are made of cations and anions joined together through an ionic bond. Cations are metals that transfer electrons....
What is a normal salt?
Normal salts: A salt that does not contain any replaceable hydrogen ions is a normal salt. It is obtained by replacing all the hydrogen (H +) ions of an acid by metal ions or ammonium ions. Normal salts are neutral. In the above equation H + in HCl is replaced by Na + .
What is a salt that contains complex ions?
A salt that contains complex ions, i.e. ions consisting of a charged group of atoms is a complex salt.
What is a salt that contains replaceable hydroxyl groups?
A salt that contains replaceable hydroxyl groups is a basic salt. It is obtained when there is an insufficient supply of acid H + ions to completely neutralize the base.
What is a salt that contains more than one type of cation or anion?
Salts that contain more than one type of cation or anion are called as mixed salts.
How are acidic salts produced?
Acidic salts are produced when each molecule of acid has more than 1 H + ion.
What is salt chemistry?
The salt definition in chemistry shows that it is an inorganic chemical that is abundantly available on the Earth's crust. It is an ionic substance composed of ions that are joined together with an electrostatic ionic bonds. An ionic salt that is commonly used by almost everyone is sodium chloride {eq}NaCl {/eq}, table salt. Most people would recognize this salt since they use it cooking and preparing meals.
What is table salt?
Sodium chloride, or table salt, is a white solid that has a small cubic form. The shape and the size of the salt is often processed and modified by subjecting the salt crystals to high temperatures.
How is a Salt Bonded Together?
A ions in a salt molecule are bonded together through an ionic bond. The metal cation transfers its electron (s) to the nonmetal anion, thus forming an ionic bond between these two ions. Taking {eq}NaCl {/eq} as an example: sodium transfers one of its outer electrons, which chlorine happily accepts. Sodium lost an electron, it has more protons than it does electrons, which makes it a cation {eq}Na^+ {/eq}. Chlorine, on the other hand, has more electrons than protons which makes it negatively charged, an anion. Taking magnesium chloride {eq}MgCl_2 {/eq} as another example: magnesium transfers two of its electrons to two chlorine atoms. Magnesium cation {eq}Mg^+2 {/eq} gave up two electrons, and each chlorine anion {eq}2Cl^- {/eq} gained a single electron. Note that magnesium is forming two ionic bonds with two chlorine atoms.
What is the chemical formula of salt?
The _hemical symbol for salt can be {eq}M^+X^- {/eq}, where M is the cation and X is the anion. The charges show which is the positive and which is the negative ion. Knowing that sodium chloride is made of sodium cations and chloride anions, their chemical formula can be easily deduced as {eq}Na^+Cl^- {/eq}. It is, however, reformatted as {eq}NaCl {/eq}. The image provided should help in deducing the molecular formula of salts. Calcium transfers two electrons, but chlorine can only accept a total of one electron (they reach the noble gas configuration after accepting 1 electron). Which is while it gives it distributes its two electrons to two chlorine atoms.
What is the bond between salt and water?
The sodium cation and chloride anion are joined together through an ionic bond . It is a type of bond that is formed when two charged atoms attract one another. Salt has a very high solubility in water, which should be expected since salt is highly polar. The water molecules pull apart the salt cations and anions, breaking their ionic bonds. The water's molecule negative part attracts the positively charged cation, and the positive part of water attracts the negatively charged chloride. Once {eq}Na^+ {/eq} and {eq}Cl^- {/eq} come apart, the water molecules surrounds them. The sodium cations are surrounded by the water's oxygens, and the chloride anions are surrounded by the hydrogens. This results in the complete dissociation of salt and the formation of a homogenous solution.
What are the colors of salt?
Salt has a crystalline structure and often appears in different colors. Calcium for instance is white, whereas copper sulfate is blue, sodium chloride ranges from transparent to white. In general, salts can have different colors depending on their purity and concentration of minerals. The melting and boiling points of salts are extremely high; an excessive amount of energy is required to break the ionic bonds of salt. Salts are polar, which makes them water soluble and excellent electricity conductors. They are good electrolytes because they completely dissociate in water, forming ions swimming in the water solution. Salts taste, well, salty, and they don't have any odor.
How are salts formed?
Salts are formed through the reaction of acids and basis. Such reactions are called neutralization reactions. The reason behind this naming is linked to the fact that when acids and basis are put together, they neutralize one another. The products of their neutralization is salt and water, both of which are neutral. Note that neutral salts are only produced when a strong acid reacts with a strong base or when a weak acid reacts with a weak base.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
Where is sodium found in the solar system?
Sodium is present in high quantities in the sun and stars . The D lines of sodium (spectral lines) are among the most prominent in the solar spectrum. They are known as Fraunhofer lines. Sodium is the seventh most abundant element on earth, comprising about 2.27% of the earth’s crust.
How much sodium is in the body?
Interestingly, 0.15% of your body is sodium.
Where Can I Buy Elemental Sodium?
You can buy sodium on Amazon, Ebay or through online element stores like Luciteria. You can usually purchase it for around $0.50 – $1 USD per gram. It is usually sold stored under oil. If you’re looking for table salt, then you can buy it at your local grocery store.
How are sodium compounds used in the paper making process?
Firstly, they’re used to produce paper. In the paper-making process, sodium hydroxide treats/separates fibers in the pulling phase. Secondly, they’re used to produce clear glass. In this process, we heat sodium carbonate with calcium oxide. Thirdly, sodium compounds are used in creating different metals. For example, sodium combined with titanium tetrachloride produces titanium metal. In another reaction, sodium is a catalyst to create artificial rubber. Similarly, there are other materials (e.x. foodstuffs and fertilizer) which involve sodium in their production/usage.
Why does sodium float on water?
To begin with, sodium floats when placed on water. This is because sodium’s density is lower than that of water’s. Subsequently, an exothermic reaction will occur, and a solution of sodium hydroxide and hydrogen gas will form. This reaction releases so much heat that the sodium melts and releases the hydrogen from the added water. Further, this released hydrogen gas can catch fire.
What is the name of the element that is flammable?
The element sodium is a soft, flammable, silvery-white metal. Sodium , named after the English word ‘soda’, has compounds which are commonly used in our day-to-day lives. You might be familiar with table salt, which is NaCl (sodium chloride), a compound of sodium. It is one of the most reactive metals on the periodic table.
How is sodium made?
Sodium is commonly made through the vector process. Producing elemental sodium in a small scale laboratory is extremely difficult, though. However, it can be done with difficulty by performing the electrolysis of molten NaCl, but this is dangerous and it’s difficult to recover the sodium.
What is sodium made of?
Sodium in the form of salt (sodium chloride, NaCl) and soda (sodium carbonate, Na2CO3) have been known since prehistoric times [1] The name of element originated from Arabic word ‘suda’ which means headache, as the association of sodium with headache was known in early times. The metal was first isolated in 1807, through the electrolysis of sodium hydroxide by Sir Humphry Davy. In 1809, Ludwig Wilhelm, a German physicist and chemist, proposed the name Natronium for it. Its chemical abbreviation ‘Na’ was published in the system of atomic symbols in 1814 which is derived from its Latin name ‘natrium’ [2].
What is the atomic mass of sodium?
Sodium has an atomic number of 1 and atomic mass of 22.98. It is placed in group 1 of periodic table as it has a single electron in its outer most shell that it readily donates, creating a positively charged ion, the Na+ cation. At room temperature Sodium is soft, silvery-white metal which can be easily cut with a knife. It is highly reactive. In air it reacts with oxygen forming grayish white sodium oxide, so it is stored in an inert liquid such as kerosene or in nitrogen gas as it does not react with nitrogen. It is lighter than water. It is a good conductor of heat and electricity and exhibits the photoelectric effect i.e. the emission of electrons when exposed to light. Sodium and its compounds give yellow color to the flame which is the basic analytical test for sodium. It burns at a temperature more than 800 °C (1,500 °F) [5].
How much sodium is needed for a healthy body?
Around 500mg of sodium is the minimum physiological requirement per day of human body. Sodium Chloride is daily used as seasoning and preservative in diet. In plants, sodium is a micronutrient which helps in metabolism. It is involved in synthesis of chlorophyll, opening and closing of stomata, and helps in uptake of water.
What is the reaction of sodium and carbon?
It is relatively less reactive with carbon, but at 625°C it reacts with carbon monoxide forming sodium carbide and sodium carbonate. With liquid ammonia sodium reacts to give blue colored solutions forming sodamide, but the reaction is quite slow. Organic acids also react with sodium to form sodium salts.
What is the importance of sodium in the cell?
Significance and Uses. Sodium is one of the most essential elements for life, as sodium and potassium keep a definite balance within the cell and are involved in maintaining an electrolyte balance across the cell membranes [6]. It helps in the regulation of blood pressure, blood volume, osmotic equilibrium and pH.
What are the characteristics of sodium hydroxide?
Chemical Characteristics. It is highly reactive in air and forms sodium hydroxide (NaOH), which is a strong base on reaction with water. In presence of air, sodium hydroxide film absorbs carbon dioxide, and lead to the formation of sodium bicarbonate. Sodium reacts with halogens under certain conditions to produce light.
How many isotopes of sodium are there?
Isotopes of Sodium. Twenty isotopes of sodium are identified, but only one, sodium-23 is stable. Two radioactive, cosmogenic isotopes which are the byproduct of cosmic ray spallation are known, Na-22 which has a half-life of 2.6 years and Na-24 with a half-life of 15 hours.
What is table salt made of?
Sea salt consists mainly of sodium chloride, with trace amounts of magnesium and calcium chlorides and sulfates, algae, sediments, and bacteria. These substances impart a complex flavor to sea salt.
Where Does Salt Come From?
One of the main sources of table salt is the mineral halite or rock salt. Halite is mined. The minerals in mined salt give it a chemical composition and flavor unique to its origin. Rock salt commonly is purified from mined halite, since halite occurs with other minerals, including some that are considered toxic. Native rock salt is sold for human consumption, but the chemical composition is not constant and there may be health risks from some of the impurities, which can be up to 15% of the mass of the product.
What are the additives in salt?
Additives to Salt. Natural salt already contains a variety of chemicals. When it is processed into table salt, it may also contain additives. One of the most common additives is iodine in the form of potassium iodide, sodium iodide, or sodium iodate. Iodized salt may also contain dextrose (a sugar) to stabilize the iodine.
Why is salt iodized?
Salt is iodized to help prevent cretinism in children as well as hypothyroidism and goiter in adults.
What is the most common household chemical?
She has taught science courses at the high school, college, and graduate levels. Table salt is one of the most common household chemicals. Table salt is 97% to 99% sodium chloride, NaCl. Pure sodium chloride is an ionic crystal solid. However, other compounds are present in table salt, depending on its source or additives ...
Why is folic acid added to salt?
Folic acid or folicin is added to help prevent neural tube defects and anemia in developing infants. This type of salt may be used by pregnant women to help prevent common birth defects. Folicin-enriched salt has a yellowish color from the vitamin.
Is sodium chloride a solid?
Pure sodium chloride is an ionic crystal solid. However, other compounds are present in table salt, depending on its source or additives that may be included before packaging. In its pure form, sodium chloride is white. Table salt may be white or may have a faint purple or blue tinge from impurities. Sea salt may be dull brown or gray.
