
What are some interesting facts about the periodic table?
Interesting Facts On Periodic Table of Elements
- Founder of Periodic Table. Dmitri Mendeleyev is the father of the modern periodic table of elements. ...
- Columns of the Periodic Table. The periodic table has 18 vertical columns called groups and seven horizontal columns called Periods.
- Size of the Atom. ...
- Unique Elements. ...
- Properties of Elements. ...
- Facts About Hydrogen. ...
What is so great about the periodic table?
To summarize, the periodic table is important because it is organized to provide a great deal of information about elements and how they relate to one another in one easy-to-use reference. The table can be used to predict the properties of elements, even those that have not yet been discovered.
What are two trends in the periodic table?
Periodic Trends of Properties of Elements In Periodic Table
- Atomic Radius
- Ionisation energy
- Electron affinity
- Electronegativity
- Valence electrons
- Valency
- Metallic character of the elements
- Non – metallic character of the elements
- Reactivity of elements
- Melting and boiling points of elements
What are the advantages of the periodic table?
ADVANTAGES OF THE PERIODIC TABLE: It is easier to remember the properties of an element if its position in the periodic table is known. The periodic table has made the study of chemistry systematic and easy. It acts as an aid to memory. The type of compounds formed by an element can be predicted by knowing it position in the periodic table.
Is Te a metal or nonmetal?
Tellurium is a semimetallic, lustrous, crystalline, brittle, silver-white element. It is usually available as a dark grey powder, it has the properties both of the metals and the non metals. Tellurium forms many compounds corresponding to those of sulfur and selenium.
Is there an element te?
52Tellurium / Atomic number
What can tellurium be used for?
Tellurium is often used to improve the machinability of copper and stainless steel. It's used to make blasting caps, added to cast iron and used in ceramics. Adding tellurium to lead improves the strength and hardness of the metal and decreases corrosion. Many thermoelectric devices are made with bismuth telluride.
Is tellurium toxic?
► Exposure to Tellurium can cause headache, fatigue, dizziness, drowsiness and weakness. ► Repeated exposure can cause garlic odor to the breath, nausea, vomiting, loss of appetite and upset stomach, metallic taste and irritability.
Why is tellurium so rare?
Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.
How much is tellurium worth?
All Metal PricesMetalPriceDateUpdatedTellurium$67.530 kg10/12Oct 12, 2022Tin$8.9902 lb10/12Oct 12, 2022Uranium$49.500 lb10/12Oct 12, 2022Zinc$1.3270 lb10/13Oct 13, 202219 more rows
Is tellurium a rare earth?
Tellurium is one of the least common elements on Earth. Most rocks contain an average of about 3 parts per billion tellurium, making it rarer than the rare earth elements and eight times less abundant than gold.
Can you touch tellurium?
* [added 1430 on 2 March 2012] Tellurium is "[h]ighly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact.
What are 2 interesting facts about tellurium?
Tellurium: Tellurium is silver/white, rare, brittle, toxic. Fun fact about Tellurium: Tellurium is one of the rarest elements on Earth, but plentiful in space. The rarity of this element is comparable to that of platinum. It is present in the earths crust in about 0.001 parts per million.
What is the rarest stable element on Earth?
Astatine is the rarest element on Earth; only approximately 25 grams occur naturally on the planet at any given time. Its existence was predicted in the 1800s, but was finally discovered about 70 years later. Decades after its discovery, very little is known about astatine.
What foods contain tellurium?
Tellurium is ingested with foods such as nuts, fish, and certain dairy products. Many fatty foods contain tellurium, and some plants, like garlic, accumulate tellurium from the soil. Neither drinking water nor ambient air contains significant amounts of tellurium.
Where is tellurium found in nature?
Tellurium is found free in nature, but is most often found in the ores sylvanite (AgAuTe4), calaverite (AuTe2) and krennerite (AuTe2). Today, most tellurium is obtained as a byproduct of mining and refining copper. Tellurium is a semiconductor and is frequently doped with copper, tin, gold or silver.
Is Te a metalloid?
Tellurium is a chemical element with symbol Te and atomic number 52. Classified as a metalloid, Tellurium is a solid at room temperature.
How many neutrons are in te?
76NameTelluriumNumber of Neutrons76Number of Electrons52Melting Point449.5° CBoiling Point989.8° C9 more rows
How many neutrons are in this element TE?
Te is the most common isotope, having a natural abundance of approximately 34%. Tellurium-120 is composed of 52 protons, 68 neutrons, and 52 electrons. Tellurium-122 is composed of 52 protons, 70 neutrons, and 52 electrons. Tellurium-123 is composed of 52 protons, 71 neutrons, and 52 electrons.
What is thallium symbol?
TlThallium / Symbolthallium (Tl), chemical element, metal of main Group 13 (IIIa, or boron group) of the periodic table, poisonous and of limited commercial value.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
What is PubChem working with?
PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What is the electron configuration of a neutral atom?
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered 1, 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram. The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
How many electrons are in neon?
The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to ...
How many categories are there in the periodic table?
The elements of the periodic table shown here are divided into nine categories; six for the metals, and two for nonmetals, and a metalloid category. The nine categories (or sets) correspond to those found in the literature for the applicable part of the periodic table. Different authors may use different categorisation schema depending on the properties of interest.
What is the Periodic Table?
The periodic table is an arrangement of all the elements known to man in accordance with their increasing atomic number and recurring chemical properties. They are assorted in a tabular arrangement wherein a row is a period and a column is a group.
How many elements are naturally occurring in the periodic table?
Therefore, as the energy level of the atom increases, the number of energy sub-levels per energy level increases. The first 94 elements of the periodic table are naturally occurring, while the rest from 95 to 118 have only been synthesized in laboratories or nuclear reactors.
What is the difference between the modern periodic law and the Mendeleev periodic table?
Mendeleev modeled his periodic table on the basis of increasing atomic mass, whereas, the modern periodic law is based on the increasing order of atomic numbers. Even though Mendeleev’s periodic table was based on atomic weight, he was able to predict the discovery and properties of certain elements. During his time only around half of the elements ...
How are elements arranged in order?
Elements are arranged from left to right and top to bottom in the order of their increasing atomic numbers. Thus,
When was Mendeleev's periodic table published?
Mendeleev’s Periodic Table was published in the German Journal of chemistry in 1869 .
When was iodine discovered?
Iodine was discovered by Bernard Courtois in 1811.
What is the periodic table?
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, ...
When the chemical elements are thus arranged, there is a recurring pattern called the periodic law?
When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. The initial discovery, which was made by Dmitry I. Mendeleyev in the mid-19th century, has been of inestimable value in the development of chemistry.
What is the atomic number of an element?
The atomic number of an element is the number of protons in the nucleus of an atom of that element . Hydrogen has 1 proton, and oganesson has ...
What elements are triads?
Döbereiner in 1817 showed that the combining weight, meaning atomic weight, of strontium lies midway between those of calcium and barium, and some years later he showed that other such “ triads ” exist (chlorine, bromine, and iodine [halogens] and lithium, sodium, and potassium [alkali metals]). J.-B.-A. Dumas, L. Gmelin, E. Lenssen, Max von Pettenkofer, and J.P. Cooke expanded Döbereiner’s suggestions between 1827 and 1858 by showing that similar relationships extended further than the triads of elements, fluorine being added to the halogens and magnesium to the alkaline-earth metals, while oxygen, sulfur, selenium, and tellurium were classed as one family and nitrogen, phosphorus, arsenic, antimony, and bismuth as another family of elements.
Why do the elements in the periodic table have different orbits?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion principle, no more than two electrons can fill the same orbital. The first row of the periodic table consists of just two elements, hydrogen and helium. As atoms have more electrons, they have more orbits available to fill, and thus the rows contain more elements farther down in the table.
How many protons does hydrogen have?
The atomic number of an element is the number of protons in the nucleus of an atom of that element. Hydrogen has 1 proton, and oganesson has 118.
What are the elements that are related to the first seven?
Newlands proposed classifying the elements in the order of increasing atomic weights, the elements being assigned ordinal numbers from unity upward and divided into seven groups having properties closely related to the first seven of the elements then known: hydrogen, lithium, beryllium, boron, carbon, nitrogen, and oxygen . This relationship was termed the law of octaves, by analogy with the seven intervals of the musical scale.
What is the value of an atom in the periodic table?
However, the value given in the periodic table is an average of the mass of all isotopes of a given element. While the number of electrons does not contribute significant mass to an atom, isotopes have differing numbers of neutrons, which do affect mass.
What is the atomic number of an element?
Element Atomic Number. One number you will find on all periodic tables is the atomic number for each element. This is the number of protons in the element, which defines its identity. How to Identify It: There isn't a standard layout for an element cell, so you need to identify the location of each important number for the specific table.
Why do periodic tables not have periods?
Most periodic tables do not number them because they are fairly obvious, but some tables do. The period indicates the highest energy level att ained by electrons of an atom of the element in the ground state. How to Identify It: Period numbers are located on the left-hand side of the table. These are simple integer numbers.
Why do periodic tables omit electron configuration?
Most tables omit this value because it takes up a lot of room.
What is the lowest atomic number?
The atomic number is easy because it is an integer that increases as you move from left to right across the table. The lowest atomic number is 1 ( hydrogen ), while the highest atomic number is 118. Examples: The atomic number of the first element, hydrogen, is 1. The atomic number of copper is 29.
How to identify atomic mass?
How to Identify It: The atomic mass is a decimal number. The number of significant figures varies from one table to another. It's common to list values to two or four decimal places. Also, the atomic mass is recalculated from time to time, so this value may change slightly for elements on a recent table compared with an older version.
How to identify an element group?
How to Identify It: The number for the element group is cited above the top element of each column. The element group values are integers running from 1 to 18.
How many elements are in the periodic table?
The table below consists of 118 elements of the periodic table, sorted by atomic number, atomic weight, symbols, density, discovered year and the group.
Who created the modern periodic table?
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley, which states that “the properties of elements are periodic functions of their atomic numbers”.
What is the atomic number of an element?
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of [He] 2s 2 2p 2, since its atomic number is 6.
What is the number of protons in the nucleus called?
The number of protons in the nucleus is called the atomic number. The atomic number of each element is unique.
Why is the atomic number of each element unique?
While the atomic number always stays the same some elements have atoms with different atomic mass numbers. This is because some elements have a different number of neutrons in the nucleus.
How to find the mass of an element?
The number of protons and the number of neutrons shall determine the mass number of an element. Since the isotopes of an element have slightly different mass numbers, it calculates the atomic mass by obtaining the mean of the mass numbers for its isotopes.
How can periodic trends be observed?
Periodic trends in the properties of the elements can be observed down the groups and across the periods of the modern periodic table. Every chemical element has a specific atomic number, which provides insight into the number of protons present within its nucleus.
How to read the periodic table?
To read the periodic table, start at the top left with the elements with the lowest atomic numbers, which tells you how many protons each atom has. Then, as you move right across the chart, make note that the atomic weight, shown at the bottom of the square, also increases.
Where is the symbol for an element?
Recognize the element’s 1 to 2-letter symbol. It most often appears in the center of the box in a large font. The symbol abbreviates the element's name, which is standardized across different languages. When you're doing experiments or working with elemental equations, you'll likely use the elements' symbols, so it's important to familiarize yourself with them.
Why does the atomic mass increase as you move down the table?
The atomic mass increases as you move across or down the table because the mass is calculated by adding up the protons and neutrons in each element’s atom. The number of protons increases with each element, which means the weight goes up, as well.
How are elements ordered?
The elements are ordered by their atomic numbers, which increase as you move across and down the periodic table. The atomic number is how many protons the element’s atom possesses. You’ll also notice that each element’s atomic mass increases as you move across the table.
Why does the periodic table have gaps?
Since elements don’t always fall neatly into groupings as they increase in number, the periodic table contains gaps. For example, the first 3 rows have gaps, as the Transition Metals don’t appear on the table until atomic number 21.
Why do most elements have atomic weights that include decimals?
Because the weights are averaged, most elements will have atomic weights that include decimals.
Where is the atomic mass of an element found?
As such, lighter atomic mass is found in the upper left corner of the table and increases with atomic number moving to the right and down the table.
What are the columns of the periodic table?
Columns of the periodic table typically mark groups or families. Three systems have been used to number families and groups: The older IUPAC system used Roman numerals together with letters to distinguish between the left (A) and right (B) side of the periodic table.
Which family of elements contains transition metals?
The lanthanide and actinide series below the body of the periodic table are transition metals, too. Todd Helmenstine. The largest family of elements consists of transition metals. The center of the periodic table contains the transition metals, plus the two rows below the body of the table (lanthanides and actinides) are special transition metals.
What family are alkali metals in?
Alkali Metals or Group 1 Family of Elements. The highlighted elements of the periodic table belong to the alkali metal element family. Todd Helmenstine. The alkali metals are recognized as a group and family of elements. These elements are metals. Sodium and potassium are examples of elements in this family.
What is the carbon group?
Carbon Group or Tetrels Family of Elements. The highlighted elements belong the carbon family of elements. These elements are collectively known as the tetrels. Todd Helmenstine. The carbon group is made up of elements called tetrels, which refers to their ability to carry a charge of 4. Group 14 or IVA.
What family does the highlighted element belong to?
The highlighted elements of this periodic table belong to the alkaline earth element family. Todd Helmenstine
How are elements categorized?
Elements may be categorized according to element families. Knowing how to identify families, which elements are included, and their properties helps predict behavior of unknown elements and their chemical reactions.
What is the Boron family?
Boron Group or Earth Metal Family of Elements. These are the elements belonging to the boron family. Todd Helmenstine. The boron group or earth metal family is not as well-known as some of the other element families. Group 13 or IIIA. Boron Group or Earth Metals.
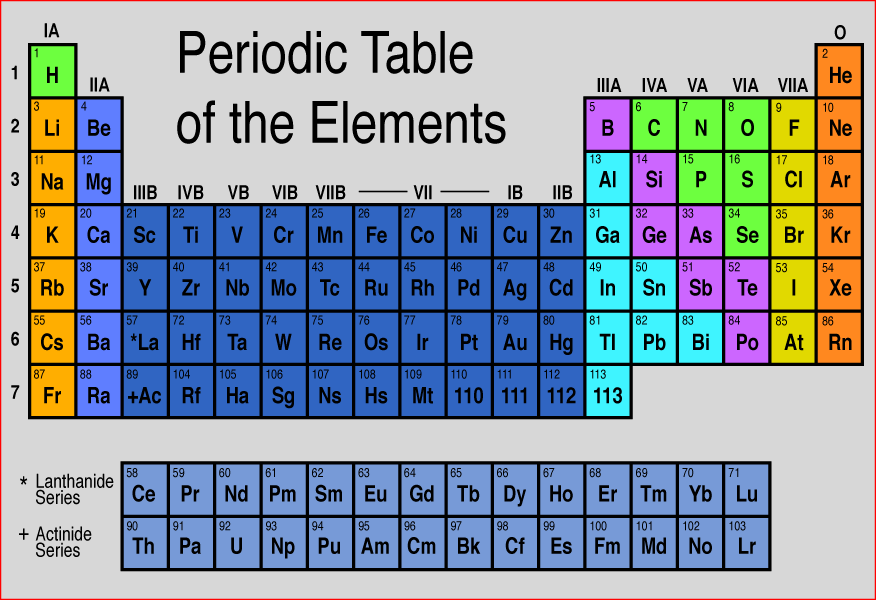
Summary
Overview
The periodic table is a 2-dimensional structured table. The elements are placed in table cells, in reading order of ascending atomic number. The table columns are called groups, the rows are called periods. The breaks at the end of each period occur according to a repetition (or periodicity) of physical and chemical properties of the elements.
Periodic trends
As chemical reactions involve the valence electrons, elements with similar outer electron configurations may be expected to react similarly and form compounds with similar proportions of elements in them. Such elements are placed in the same group, and thus there tend to be clear similarities and trends in chemical behaviour as one proceeds down a group. As analogous configurations return …
Classification of elements
Many terms have been used in the literature to describe sets of elements that behave similarly. The group names alkali metal, alkaline earth metal, pnictogen, chalcogen, halogen, and noble gas are acknowledged by IUPAC; the other groups can be referred to by their number, or by their first element (e.g., group 6 is the chromium group). Some divide the p-block elements from groups 13 to …
History
In 1817, German physicist Johann Wolfgang Döbereiner began to formulate one of the earliest attempts to classify the elements. In 1829, he found that he could form some of the elements into groups of three, with the members of each group having related properties. He termed these groups triads. Chlorine, bromine, and iodine formed a triad; as did calcium, strontium, and barium; lithi…
Current questions
Although the modern periodic table is standard today, some variation can be found in period 1 and group 3. Discussion is ongoing about the placements of the relevant elements. The controversy has to do with conflicting understandings of whether chemical or electronic properties should primarily decide periodic table placement, and conflicting views of how the evidence should be used. A similar potential problem has been raised by theoretical investigations of the superheav…
Future extension beyond the seventh period
The most recently named elements – nihonium (113), moscovium (115), tennessine (117), and oganesson (118) – completed the seventh row of the periodic table. Future elements would have to begin an eighth row. These elements may be referred to either by their atomic numbers (e.g. "element 119"), or by the IUPAC systematic element names which directly relate to the atomic …
Alternative periodic tables
The periodic law may be represented in multiple ways, of which the standard periodic table is only one. Within 100 years of the appearance of Mendeleev's table in 1869, Edward G. Mazurs had collected an estimated 700 different published versions of the periodic table. Many forms retain the rectangular structure, including Janet's left-step periodic table (pictured below), and the m…