How to calculate the first ionization energy?
How to Calculate Ionization Energy. Ionization potential for hydrogen can be calculated using the following equation: E = hcRH (1/n 2 ), where. E is energy of the electron (or the amount of energy it takes to remove the electron, ionization energy) h is Planck’s constant = 6.626 * 10 -34 Js (joules*seconds)
How do you calculate second ionization energy?
∴ Order of second ionization enthalpy is O>F>N>C. How do you calculate second ionization energy? Second ionisation energy is defined by the equation: It is the energy needed to remove a second electron from each ion in 1 mole of gaseous 1+ ions to give gaseous 2+ ions.
What trend in ionization energy occurs across a period on the periodic table?
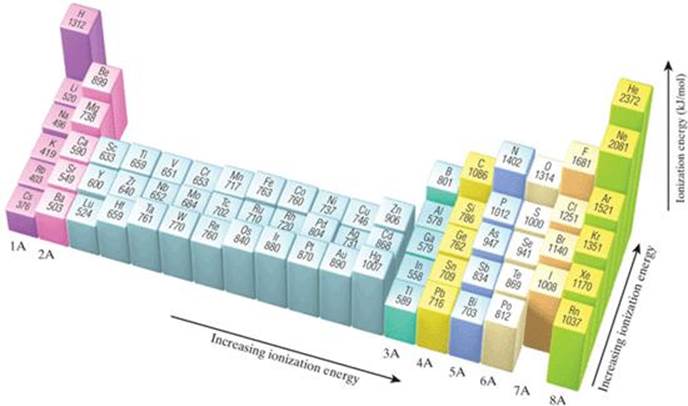
Ionization energy exhibits periodicity on the periodic table. The general trend is for ionization energy to increase moving from left to right across an element period. Moving left to right across a period, atomic radius decreases, so electrons are more attracted to the (closer) nucleus.
What trend does the first ionization energy follow?
What is the trend in first ionization energy down a group? On the periodic table, first ionization energy generally decreases as you move down a group. This is because the outermost electron is, on average, farther from the nucleus, meaning it is held less tightly and requires less energy to remove.
What is the periodic trend for first ionization energy?
The first ionization energy varies in a predictable way across the periodic table. The ionization energy decreases from top to bottom in groups, and increases from left to right across a period.
What is the group trend in the first ionization energies Why?
On the periodic table, first ionization energy generally decreases as you move down a group. This is because the outermost electron is, on average, farther from the nucleus, meaning it is held less tightly and requires less energy to remove.
What is the trend for first ionization energy in groups and across periods?
In general, ionization energy increases across a period and decreases down a group. Across a period, effective nuclear charge increases as electron shielding remains constant.
What is the trend in ionization energy?
The ionization energy of the elements within a period generally increases from left to right. This is due to valence shell stability. The ionization energy of the elements within a group generally decreases from top to bottom. This is due to electron shielding.
What is the trend in first second and third ionization energies?
The third ionization energy is the energy it takes to remove an electron from a 2+ ion. (That means that the atom has already lost two electrons, you are now removing the third.) And 2nd ionization energy is higher than 1st ionization energy, 3rd is higher than 2nd, and so forth.
What are 1st 2nd and 3rd ionization energies?
0:3210:591st 2nd 3rd ionization - YouTubeYouTubeStart of suggested clipEnd of suggested clipGeneral trend in this is that the first ionization energy will always be the last and secondMoreGeneral trend in this is that the first ionization energy will always be the last and second ionization energy is always less than the third ionization.
What is the trend for second ionization energy?
Second ionization energy decreases as you go down the group. Third ionization energy decreases as you go down the group. For each element in the Group, the first ionization energy is less than the second ionization energy which is less than the third ionization energy.
Which is the correct order of ionization energies?
Correct Answer: Option B. Explanation: Since it is easier to take out an electron from a negatively charged ion than from a neutral atom, the ionization energies for anions will be lesser than the corresponding neutral atoms. So the order of ionisation energy is F>F− and Cl>Cl−.
What is the trend across a period?
The atomic radius across a period in the periodic table tends to decrease from left to right. As you move across a period the number of electrons from one element to the next increases by one.
What is the general trend in first ionization energy and electronegativity?
The first ionization energy decreases and the electronegativity decreases.
How does ionization energy change across a period?
Ionization energies increase across a Period, and decrease down a Group.
Why is the first ionization energy lower than the second?
Because positive charge binds electrons more strongly, the second ionization energy of an element is always higher than the first.
Why do group 1 elements have the highest second ionization energy?
Alkali metals acquire, noble gas configuration after losing 1 electron, therefore their second ionization energy is higher than alkaline earth metals.
What is the trend in group 1 elements?
Explaining trends The reactivity of Group 1 elements increases as you go down the group because: the atoms get larger as you go down the group. the outer electron gets further from the nucleus as you go down the group.
What is the trend going down group 1?
Group 1 elements are known as Alkali Metals. They are called s-block elements because their highest energy electrons appear in the s subshell. Progressing down group 1, the atomic radius increases due to the extra shell of electrons for each element. Going down the group, the first ionisation energy decreases.
Why is second ionisation energy higher group 1?
We remove electrons from a neutral atom in the first ionization energy, but in the second ionization energy, we must remove electrons from a positive atom, where electrons are more securely bound due to increased attraction force, hence second ionization energy is higher than the first.
What is the trend of ionization?
Ionization, together with atomic and ionic radius, electronegativity, electron affinity, and metallicity, follows a trend on the periodic table of elements. Ionization energy generally increases moving from left to right across an element period ( row). This is because the atomic radius generally decreases moving across a period, ...
What is the trend for ionization energy to decrease moving from top to bottom down a periodic table group?
The general trend is for ionization energy to decrease moving from top to bottom down a periodic table group. Moving down a group, a valence shell is added. The outermost electrons are further from the positive-charged nucleus, so they are easier to remove.
Why does ionization decrease as electrons move down the group?
This is because the principal quantum number of the outermost electron increases moving down a group. There are more protons in atoms moving down a group (greater positive charge), yet the effect is to pull in the electron shells, making them smaller and screening outer electrons from the attractive force of the nucleus. More electron shells are added moving down a group, so the outermost electron becomes increasingly distance from the nucleus.
What is the energy required to remove an electron from a gaseous atom?
Updated July 03, 2019. Ionization energy is the energy required to remove an electron from a gaseous atom or ion. The first or initial ionization energy or E i of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions.
What is ionization energy?
Ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. The most common units of ionization energy are kilojoules per mole (kJ/M) or electron volts (eV). Ionization energy exhibits periodicity on the periodic table. The general trend is for ionization energy to increase moving from left ...
What are the exceptions to the ionization energy trend?
Exceptions to the Ionization Energy Trend. If you look at a chart of first ionization energies, two exceptions to the trend are readily apparent. The first ionization energy of boron is less than that of beryllium and the first ionization energy of oxygen is less than that of nitrogen.
Why is ionization energy important?
Ionization energy is important because it can be used to help predict the strength of chemical bonds. Also Known As: ionization potential, IE, IP, ΔH°. Units: Ionization energy is reported in units of kilojoule per mole (kJ/mol) or electron volts (eV).
Why does Cu have a higher ionization energy than Zn?
Since it is a transition metal and has completely filled s and d orbitals, it gains extra stability and it becomes tougher to remove a valence electron from Zinc. This is why the First ionization energy shoots up from Cu ( 746 kJ/ mol) to Zn (906 kJ/mol).
Which element has a lower ionization energy than Arsenic?
c) Selenium – Selenium has a first ionization energy lesser than that of Arsenic. Arsenic has electronic configuration [Ar]3d10 4s2 4p3 . Arsenic already has the stable configuration of half-filled sublevel so it wouldn’t give up it’s electron as readily, so more energy is required to remove it.
What happens to the valence shell in period 4?
For example, in period 4, each electron is added to the last, i.e., 4th or N energy level. The shielding effect remains the same since the number of completely filled inner shells remains the same. The concentration of more protons in the nucleus combined with the above 2 effects, causes effective nuclear charge to increase ; pulling the valence shell more tightly towards the nucleus and causing atomic size to shrink. Therefore, the electrons are more tightly held, it requires more energy to remove them. Since first ionization energy is a measure of the energy required to remove a valence electron, it increases as more energy is required.
What is the effect of the Schrodinger wave on the atomic size of a francium atom?
This causes atomic size to decrease and increases the attraction between the valence shell and the nucleus. Shielding effect also decreases considerably due to the loss of one whole fully filled inner shell (Energy level 6).
Which has a lower ionization energy, Selenium or Arsenic?
But, in the 4p sublevel of Selenium, there is pairing in the 4px orbital, increasing electron – electron repulsion, making it easier to remove an electron from Selenium than from Arsenic. Hence, selenium has a lower ionization energy than Arsenic.
How long did it take for quantum theory to develop?
Although both theories revolutionized the world of physics, the Quantum Theory required a period of over three decades to develop, while the Special Theory of Relativity was created in a single year. The development ...
What are the two forms of energy?
The Essay on What are the forms of energy. Energy comes in two basic forms: potential and kinetic. Potential Energy – is any type of stored energy; it is not shown through movement. Potential energy can be chemical, nuclear, gravitational, or mechanical.
What period does the graph show how the first ionisation energy varies across?
The graph shows how the first ionisation energy varies across period 3.
What is the first ionisation energy?
First ionisation energy is the enthalpy change when one mole of gaseous atoms forms one mole of gaseous ions with a single positive charge. It is an endothermic process, i.e. Δ H is positive. A general equation for this enthalpy change is: X (g) → X + (g) + e –.
