What period does nitrogen belong to?
Nitrogen's location in the periodic table of the elements. Todd Helmenstine. Nitrogen is the seventh element on the periodic table. It is located in period 2 and group 15. It is at the top of the periodic table, between carbon and oxygen.
Is nitrogen in the same period as phosphorus?
• Nitrogen is in the second period, whereas phosphorus is in the third period. • Naturally nitrogen occurs as a diatomic gas, whereas phosphorus occurs in solid state. • Phosphorus has the capability to make bonds until it has more than an octet in the valence shell. But nitrogen forms bonds until an octet is filled.
What is happening in the nitrogen cycle?
The nitrogen cycle contains several stages:
- Nitrogen fixation. Atmospheric nitrogen occurs primarily in an inert form (N2) that few organisms can use; therefore it must be converted to an organic – or fixed – form in ...
- Nitrification.
- Assimilation.
- Ammonification.
- Denitrification.
What period and group is carbon in?
Carbon (from Latin: carbo "coal") is a chemical element with symbol C and atomic number 6. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table.

Is nitrogen a period 2?
The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon.
What group is nitrogen in?
Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi).
Where is period 2 on the periodic table?
The horizontal rows of the periodic table are called periods. Period 2, or the second period, refers to the second row from the top of the periodic table.
What type of element is nitrogen?
nonmetal chemical elementNitrogen, N, is a nonmetal chemical element with 7 protons. It is present in all living tissues as proteins, nucleic acids, and other molecules, and is the largest single component of the Earth's atmosphere.
Why is nitrogen in Group 15?
In the periodic table, each of the nitrogen group elements occupies the fifth position among the main group elements of its period, a position designated 15. In terms of the electronic configuration of its atoms, each nitrogen group element possesses an outermost shell of five electrons.
What element is in Group 4 Period 5?
ZirconiumThe symbol of the element in Group 4 and Period 5 is Zr. It is the symbol of Zirconium. Was this answer helpful?
What element is in period 3?
The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block.
Which elements in period 2 are metals?
The elements in the second period are Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine and neon. Among this, Lithium and Beryllium are the elements that are metals.
How do you find the period of the periodic table?
If you are given with the atomic number of an element you can find it's period number and group number. The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons.
What is nitrogen known as?
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System.
What is element 7 on the periodic table?
N NitrogenThe Elements, sorted by Atomic NumberAtomic NumberSymbolName6CCarbon7NNitrogen8OOxygen9FFluorine76 more rows
What are the 7 periodic properties?
7. S: Periodic Properties of the Elements (Summary)7.1: Development of the Periodic Table.7.2: Effective Nuclear Charge.7.3: Sizes of Atoms and Ions.7.4: Ionization Energy.7.5: Electron Affinities.7.6: Metals, Nonmetals, and Metalloids.7.7: Group Trends for the Active Metals.7.8: Group Trends for Selected Nonmetals.
What is Group 14 called?
carbon group element, any of the six chemical elements that make up Group 14 (IVa) of the periodic table—namely, carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl).
What is Group 16 called?
oxygen group element, also called chalcogen, any of the six chemical elements making up Group 16 (VIa) of the periodic classification—namely, oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po), and livermorium (Lv).
What is Group 15 called?
the Nitrogen familyGroup 15 elements are also called the Nitrogen family. It includes nitrogen, phosphorus, arsenic, antimony and bismuth elements.
What is nh2 group called?
aminesThe substituent −NH 2 is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure R−CO−NR′R″, are called amides and have different chemical properties from amines.
What is the temperature of nitrogen?
Liquid nitrogen (made by distilling liquid air) boils at 77.4 kelvins (−195.8°C) and is used as a coolant.
What is the atomic radius of nitrogen?
The atomic radius of Nitrogen atom is 71pm (covalent radius).
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N.
What is the crystal structure of nitrogen?
A possible crystal structure of Nitrogen is hexagonal structure .
What is the first ionization energy of nitrogen?
First Ionization Energy of Nitrogen is 14.5341 eV.
What is the electron configuration of nitrogen?
Electron configuration of Nitrogen is [He ] 2s2 2p3.
What is the electronegativity of nitrogen?
The electronegativity of Nitrogen is: χ = 3.04
What group is nitrogen in?
Nitrogen element is in group 15 and period 2 of the Periodic table. Nitrogen is the p-block element and it belongs to the category of Nonmetals.
How much nitrogen is in the atmosphere?
Because of these reasons, nitrogen is 78 % in Earth’s atmosphere.
Why is Nitrogen an important element for sustaining life?
Nitrogen element is very important for sustaining life because of the following reasons.
How many electrons does nitrogen have?
Nitrogen atom has 5 electrons in its outermost orbit.
How much oxygen is in the air we breathe?
The air which we breathe in contains 78% of nitrogen and 21% oxygen.
What is the electron configuration of nitrogen?
For example; the electron configuration of nitrogen is [He] 2s 2 2p 3.
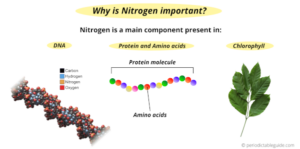
Where is nitrogen found in the body?
Nitrogen is present in the nucleic acid of our body which includes DNA and RNA. DNA is responsible for the transfer of genetic information from one generation to another.
Who first discovered nitrogen?
Nitrogen first was considered a chemical element by Antoine-Laurent Lavoisier, whose explanation of the role of oxygen in combustion eventually overthrew the phlogiston theory, an erroneous view of combustion that became popular in the early 18th century.
Where is nitrogen found in the Earth?
In combination, nitrogen is found in the rain and soil as ammonia and ammonium salts and in seawater as ammonium (NH 4+ ), nitrite (NO 2− ), and nitrate (NO 3−) ions. Nitrogen constitutes on the average about 16 percent by weight of the complex organic compounds known as proteins, present in all living organisms. The natural abundance of nitrogen in Earth’s crust is 0.3 part per 1,000. The cosmic abundance—the estimated total abundance in the universe—is between three and seven atoms per atom of silicon, which is taken as the standard.
What are some reactions that yield nitrogen?
Various laboratory reactions that yield nitrogen include heating ammonium nitrite (NH 4 NO 2) solutions, oxidation of ammonia by bromine water, and oxidation of ammonia by hot cupric oxide. Elemental nitrogen can be used as an inert atmosphere for reactions requiring the exclusion of oxygen and moisture.
What is nitrogen used for?
In the electrical industry nitrogen is used to prevent oxidation and other chemical reactions, to pressurize cable jackets, and to shield motors. Nitrogen finds application in the metals industry in welding, soldering, and brazing, where it helps prevent oxidation, carburization, and decarburization.
How is nitrogen made?
Commercial production of nitrogen is largely by fractional distillation of liquefied air. The boiling temperature of nitrogen is −195.8 °C (−320.4 °F), about 13 °C (−23 °F) below that of oxygen, which is therefore left behind. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. On a small scale, pure nitrogen is made by heating barium azide, Ba (N 3) 2. Various laboratory reactions that yield nitrogen include heating ammonium nitrite (NH 4 NO 2) solutions, oxidation of ammonia by bromine water, and oxidation of ammonia by hot cupric oxide.
What is the name of the gas in the atmosphere?
About four-fifths of Earth’s atmosphere is nitrogen, which was isolated and recognized as a specific substance during early investigations of the air. Carl Wilhelm Scheele, a Swedish chemist, showed in 1772 that air is a mixture of two gases, one of which he called “fire air,” because it supported combustion, and the other “foul air,” because it was left after the “fire air” had been used up. The “fire air” was, of course, oxygen and the “foul air” nitrogen. At about the same time, nitrogen also was recognized by a Scottish botanist, Daniel Rutherford (who was the first to publish his findings), by the British chemist Henry Cavendish, and by the British clergyman and scientist Joseph Priestley, who, with Scheele, is given credit for the discovery of oxygen. Later work showed the new gas to be a constituent of nitre, a common name for potassium nitrate (KNO 3 ), and, accordingly, it was named nitrogen by the French chemist Jean-Antoine-Claude Chaptal in 1790. Nitrogen first was considered a chemical element by Antoine-Laurent Lavoisier, whose explanation of the role of oxygen in combustion eventually overthrew the phlogiston theory, an erroneous view of combustion that became popular in the early 18th century. The inability of nitrogen to support life (Greek: zoe) led Lavoisier to name it azote, still the French equivalent of nitrogen.
What is the source of nitrogen?
Among the elements, nitrogen ranks sixth in cosmic abundance. The atmosphere of Earth consists of 75.51 percent by weight (or 78.09 percent by volume) of nitrogen; this is the principal source of nitrogen for commerce and industry. The atmosphere also contains varying small amounts of ammonia and ammonium salts, as well as nitrogen oxides and nitric acid (the latter substances being formed in electrical storms and in the internal combustion engine). Free nitrogen is found in many meteorites; in gases of volcanoes, mines, and some mineral springs; in the Sun; and in some stars and nebulae.
What is the alpha phase of nitrogen?
The alpha phase is another allotropic form of nitrogen that it acquires by arranging in a cubic crystal when exposed to temperature lower than -237C. The density of liquid nitrogen is 0.808 g/mL which is about 80.8% denser than water.
Who discovered nitrogen?
Nitrogen was discovered as a novel element by Daniel Rutherford (1722) and he termed it as the noxious air [2]. He found that it was that component of the air that did not support combustion.
What is the Greek word for nitrogen?
Earlier, Antoine Lavoisier used the term azote (Greek) meaning no life for nitrogen, which later became choke or to suffocate and the term pnictogens (Greek for choke) was assigned to Group 15 due to nitrogen. In 1970, nitrogene (French word) was given to the element by Antoine Chaptal and in 1974 it became nitrogen in English language.
What is nitrogen used for?
Nitrogen is used to make adhesives and glues (in the form of cyanoacrylate). Nitrogen is used in to manufacture high quality stainless steel.
How many isotopes of nitrogen are there?
There are two stable isotope of nitrogen, nitrogen-14 and nitogen-15. Nitrogen-14 is very abundant and makes about 99.6% of naturally occurring nitrogen. There isotopes are produced in the stars. Nitrogen-15 was discovered in 1929 by S.M. Naude. There are ten artificially produced radioactive isotopes of nitrogen, ranging from nitrogen-12 to nitrogen-23.
When was hassium discovered?
Hassium is a synthetic element that was discovered in 1978. It is a highly radioactive…
Is nitrogen a gas?
Nitrogen is a colorless and odorless gas. Liquid nitrogen resembles water in its appearance, as it is colorless. Nitrogen is a very light gas and is the lightest gas in its group (Group 15). Molecular nitrogen undergoes liquefaction at -195.79C and freezes at -210 C and acquire a beta hexagonal structural assembly [3].
Where Is Nitrogen Found On The Periodic Table?
Nitrogen's location in the periodic table of the elements. Todd Helmenstine
What are the elements in the nitrogen family?
The other elements in the family are phosphorus, arsenic, antimony, bismuth, and moscovium. Elements in this group are characterized by having 5 valence electrons. This gives rise to their other name -- the pentels.
What is the atomic number of nitrogen?
The atomic number is the number of electrons in that element. The atomic number of nitrogen is 7. That is, the number of electrons in nitrogen is seven.
What is the electron configuration of nitrogen?
Nitrogen electron configuration is 1s 2 2s 2 2p 3. The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen (N) and the orbital diagram is the main topic of this article. Also, period and group determination, valency and valence electrons of nitrogen, various reactions and compound formation, bond formation of nitrogen have been discussed.
How to write the orbital diagram for nitrogen (N)?
And Pauli’s exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. To write the orbital diagram of nitrogen (N), you have to do the electron configuration of nitrogen. Which has been discussed in detail above.
How many unpaired electrons are in nitrogen?
Here, the electron configuration of nitrogen shows that three unpaired electrons exist. In this case, the valency of the nitrogen atom is 3.
How many electrons are in the last orbit of a nitrogen atom?
The total number of electrons in the last orbit of the nitrogen atom is five. That is, the group number of nitrogen is 5 + 10 = 15. Therefore, we can say that the period of the nitrogen element is 2 and the group is 15.
What is the seventh element in the periodic table?
The seventh element in the periodic table is nitrogen . The atomic number of nitrogen is 7 and the total number of electrons in a nitrogen atom is seven. These electrons are arranged according to specific rules of different orbits.
How to determine the group of p-block elements?
To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit.
What is the nitrogen cycle?
Nitrogen cycle. Nitrogen exists in the soil system in many forms, and changes (transforms) very easily from one form to another. The route N follows in and out of the soil system is collectively called the nitrogen cycle (Figure 1). The nitrogen cycle is biologically influenced.
How is the nitrogen cycle influenced?
Biological processes, in turn, are influenced by prevailing climatic conditions along with a particular soil’s physical and chemical properties. Both climate and soils vary greatly across Minnesota and affect N transformations for the different areas.
What are the factors that affect nitrogen?
What you should know 1 Numerous nitrogen (N) sources exist. Consider these when evaluating the N budget. 2 Soil type and climate greatly affect nitrogen loss from the soil system. 3 Because Minnesota has such diverse soils and climate, N cycle interpretations should be site-specific.
How long does it take for nitrogen to decay?
Nitrogen exists in crop residues in complex organic forms and the residue must decay – a process that can take several years – before N becomes available for plant use. Soil organic matter. Soil organic matter is also a major source of N used by crops.
What is the main step in the production of commercial nitrogen fertilizers?
Commercial N fertilizers are also derived from the atmospheric N pool. The major step is to combine N2 with hydrogen (H2) to form ammonia (NH3). Anhydrous ammonia is then used as a starting point in the manufacture of other nitrogen fertilizers.
Why understand nitrogen?
Why understand N. Environmental and economic issues have increased the need to better understand the role and fate of nitrogen (N) in crop production systems . Nitrogen is the nutrient most often deficient for crop production in Minnesota, and its use can result in substantial economic return for farmers.
How much N is in soil?
Soils contain approximately 2,000 pounds of N in organic forms for each percent of organic matter. This portion of organic matter decomposes at a rather slow rate and releases about 20 pounds of N per acre per year for each percent of organic matter.
