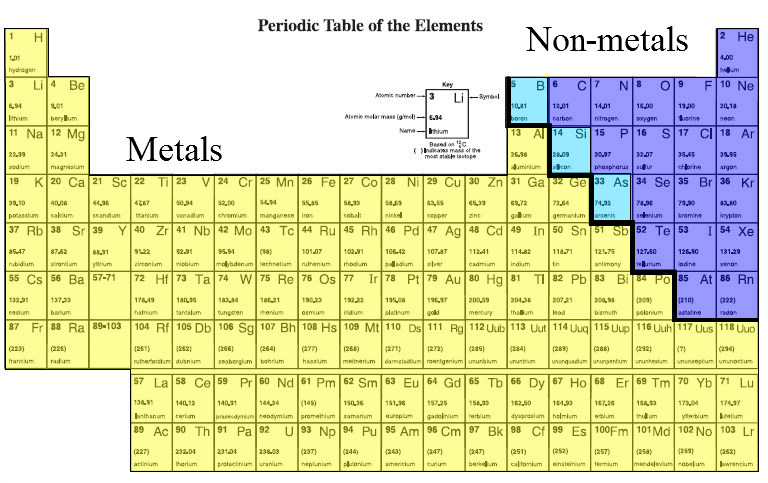
Where are metallic elements found in the periodic table?
Metals: List of Elements
- Alkali Metals. Alkali metals are in group IA on the far left side of the periodic table. ...
- Alkaline Earth Metals. The alkaline earth metals are found in group IIA of the periodic table, which is the second column of elements.
- Basic Metals. ...
- Transition Metals. ...
- More About Metals. ...
Where would nonmetals be on the periodic table?
The nonmetal elements occupy the upper right-hand corner of the periodic table. Nonmetals include the nonmetal group, the halogens, and the noble gases. These elements have similar chemical properties that differ from the elements considered metals. The nonmetal element group is a subset of these elements. The nonmetal element group consists of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur and selenium.
Where are rare elements on a periodic table?
Rare Earth Elements in Periodic Table [Image will be Uploaded Soon] Rare earth elements, also commonly known as lanthanides are basically the elements present in the top extended row placed below the main body of the periodic table and the rare earth metals stretched from Cerium (Ce) to Lutetium (Lu). The other rare earth metals are situated in ...
Where can metalloids be found on the periodic table?
The metalloids are located along a slanted line between the metal elements and nonmetal elements of the periodic table. They span from Group 13 to Group 16, 17, or 18 based on what criteria of classifying metalloid elements is being used.

Where are transition metals found?
Transition metals are found in the middle part of the Periodic table (from Group 3 to group 11).
Where are alkaline earth metals located?
Alkaline earth metals are located on the left side of the Periodic table in group 2. Alkaline earth metals are also the reactive type of metals but they are less reactive as compared to alkali metals. All the alkaline earth metals have 2 electrons in their outermost orbit.
How many electrons are in an alkaline metal?
All the alkaline earth metals have 2 electrons in their outermost orbit.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
What is the inner transition metal?
The answer is: These elements have somewhat similar properties like that of transition metals, plus they are the elements of group 3 only, but they are placed at the bottom of the Periodic table as the inner section of group 3. Hence they are known as inner transition metals.
Which metal is the most reactive?
Alkali metals are the most reactive type of metals from the entire Periodic table of elements. As we move down the group from top to bottom in the group 1, the reactivity of alkali metals increases. List of alkali metals with atomic number, symbol and name. Atomic number. Symbol.
What color is heavy metal?
These heavy metals are displayed on the Periodic table with red color (see above image)
Where is magnesium found?
Magnesium is found in its mineral magnesite.
What metals react with water?
They also react with water and moisture of the air, if they are kept open. As alkaline earth metals are reactive, they always occur in the compound form in nature. For example; Beryllium is found in its mineral beryl, chrysoberyl and phenacite. Magnesium is found in its mineral magnesite.
What happens to the metallic character of alkaline earth metals as the atomic size increases?
Down the group, the atomic size increases. And as the atomic size increases, the electron will be lost easily. So the metallic character of alkaline earth metals increases from top to bottom in a group. Read more about: Metallic character trend in periodic table.
What is the name of the metal that does not form an alkaline solution?
See the bold word “alkaline” and “earth” in the above 2 points, you will get the exact reason why they are called alkaline earth metals. ( Note: Beryllium (Be) is the only alkaline earth metal which does not form an alkaline solution on reacting with water.
What happens to electronegativity as we move down in a group?
As we move down in a group, Electronegativity of the alkaline earth metals decreases.
What is the color of alkaline earth metals?
All the alkaline earth metals are silvery-white, shiny and somewhat reactive.
How many valence electrons does an alkaline metal have?
Alkaline earth metals are similar in the following manner. All the alkaline earth metals have 2 valence electrons in their outermost orbit. They lose these two electrons and form cation with +2 charge. As you can see above that the alkaline earth metals have 2 electrons in the outermost shell.
Where are metalloids found in the periodic table?
Also we can say that metalloids are present in the diagonal region of the p block on Periodic table.
What is the color of the metalloids in the periodic table?
June 10, 2021 August 25, 2020 by Admin. Metalloids are located between the metals and nonmetals. The orange color on the Periodic table represents metalloids . They form a separating boundary between the metals and nonmetals. In other words, metalloids (semimetals) are located on the right side of the post transition metals and on the left side ...
What are the properties of metalloids?
Let us discuss the physical properties as well as chemical properties of metalloids/semimetals.
What is the chemical reaction between halogens and metalloids?
Metalloids + Halogens = Compounds (metalloid s elements reacts with halogens and finally compounds are formed by this chemical reaction) Metalloids have different metallic allotropes as well as nonmetallic allotropes. Metalloids have the property to form glasses on oxidation and so that are used in glass manufacturing.
What are elements that show some properties of metals as well as solid nonmetals called?
The elements that show some properties of metals as well as solid nonmetals are called metalloids . Metalloids look like metals, but they are not. Metalloids are brittle like solid nonmetals. Metalloids are neither conductor nor insulated. Source ( James L Marshall, Silicon, Germanium / CC BY-SA, CC BY ) The examples of metalloids are:
Why are metalloids called semiconductors?
Metalloids are called semiconductors because they are not good conductors like metals and also they are not bad conductors like nonmetals. They have the conductivity which is higher than nonmetals, but lower than metals. Hence metalloids are known as semiconductors.
How many metalloids are there?
Hence, there are total 6 known metalloids/semimetals on the Periodic table.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
Which group of periodic table is alkali metals?
Alkali metals are present in the first group of Periodic table. First group indicates that they have only one electron in their outermost orbit. As only one electron is present in the outermost orbit, it is very easy to lose or donate this electron during a chemical reaction.
Where are alkali metals located?
Alkali metals are located in group 1 on the left side of the Periodic table.
Why are alkali metals so reactive?
There are many reasons behind the reactivity of alkali metals. Let us discuss them one by one.
What metals react with water?
When these metals (Li, Na, K, Rb, Cs, Fr) react with water, they form alkalis (i.e strong base).
How many electrons are in an alkali metal?
Look, as we move down the group from top to bottom, the atomic size increases. Also alkali metals have only one electron in their outermost orbit.
Which element is the most reactive?
And hence the element with bigger atomic size (i.e Francium) is most reactive alkali metal. ( Note: Francium is a laboratory made element. It is available in a very less quantity. And hence for any practical purposes, cesium is considered as the most reactive metal in Periodic table.
Which metal decreases from top to bottom in a group?
Electronegativity of the alkali metals decreases from top to bottom in a group.
How many nonmetals are there in the periodic table?
There are 18 nonmetals on the Periodic table. All these nonmetals are located on the upper right corner of the Periodic table (Hydrogen is located on the left top corner) In the above image, the nonmetals are represented in yellow color. [ Note: Astatine (atomic number 85) shows characteristics of nonmetals (halogens) as well as metalloids.
Where is hydrogen on the periodic table?
Exception: Hydrogen is a nonmetal which is located on the left top corner of the Periodic table.
What are nonmetals?
Or in other words nonmetals are those elements which do not possess the properties of metals.
How many electrons are in the outermost orbit of a nonmetal?
Nonmetals have 4 to 8 electrons in their outermost orbit. (Halogens have 7 electrons and noble gases have 8 electrons in outermost orbit)
Why are nonmetals oxidizing agents?
Nonmetals are oxidizing agents because they gain electron/s during a chemical reaction and get reduced.
What does it mean when a solid nonmetal breaks?
Solid nonmetals are brittle in nature. That means they break easily when force is applied on them.
Which nonmetals are the most reactive?
Actually the halogens are the most reactive nonmetals, but we know that as we move down the group, the electronegativity decreases. In other words, Fluorine is at the top of the halogen group and it has less atomic size plus it needs only one electron to complete the octet.
Alkali Alloys
- The title alkali metals emanates from the Arabic expression al-qali, significance ashes. These metals are most reactive when in contact withwater and air. Alternatively, oil. They may be generally found in salts, and also a physique-focused cubic structure. As their reactivity increases, they also become more reactive in water. Within the Routine D...
Move Materials
- Inside the Regular Desk, cross over precious metals are by natural means numerous factors within the earth’s crust. These reactive factors typically kind ores with many other precious metals and non-metals. The best boiling and melting factors are associated with cross over materials. Their least expensive melting and boiling factors are linked to low-alloys. The attributes of transi…
Principal Class
- The main selection of the occasional kitchen table includes the weather with s and p electron styles. This team involves each precious metals and nonmetals. Precious metals display various attributes, which includes power conductivity, lustre, and malleability. In addition, these factors are given to get rid of electrons, and will form ions having a good cost or no fee whatsoever. Bec…
Complex Metal Alloys
- Inside the context of the trying to recycle of materials and their elements, many complicated metallic alloys consist of five or more elemental constituents. This study will likely determine all those precious metals which are not functionally reprocessed, and can gather information on end-of-daily life deficits, exploration companionality, and import dependency. The studies will establi…
Halogens
- One band of the occasional table that does not belong in the metallic loved ones are the halogens. These extremely reactive non-metal factors can be found in group of people 17 and constitute the 17th line. They can be made up of fluorine, chlorine and bromine and iodine, and as of this creating, astatine. Halogens are really unusual in the world and they are radioactive. Continue re…
Respectable Fumes
- Till lately, you could have not actually looked at the part of noble fumes in the Occasional Kitchen table. They are utilised in arc welding, inflating car tires, and lowering the solubility of real o2 for deep-seas divers. Right now, they’re even employed in explosives. Of course, if you imagined helium was dangerous, think of this: helium will be the very least reactive of all of the respectabl…
Causes of Metal Poisoning
- Although heavy metals normally occur in the environment, anthropogenic activities certainly are a major reason for pollution inside the setting. Man being exposed to heavy metals has several severe consequences, which include neurological outcomes, cardiac illnesses, developmental abnormalities, hearing loss, and malignancy. The actual character from the negative effects of c…