.png)
Where are metallic elements found in the periodic table?
Metals: List of Elements
- Alkali Metals. Alkali metals are in group IA on the far left side of the periodic table. ...
- Alkaline Earth Metals. The alkaline earth metals are found in group IIA of the periodic table, which is the second column of elements.
- Basic Metals. ...
- Transition Metals. ...
- More About Metals. ...
What are the basic metals on the periodic table?
- Element 13 - Aluminum
- Element 31 - Gallium
- Element 49 - Indium
- Element 50 - Tin
- Element 81 - Thallium
- Element 82 - Lead
- Element 83 - Bismuth
- Element 113 - Ununtrium - will probably be a basic metal.
- Element 114 - Flerovium - will probably be a basic metal.
- Element 115 - Ununpentium - will probably be a basic metal.
Where are rare elements on a periodic table?
Rare Earth Elements in Periodic Table [Image will be Uploaded Soon] Rare earth elements, also commonly known as lanthanides are basically the elements present in the top extended row placed below the main body of the periodic table and the rare earth metals stretched from Cerium (Ce) to Lutetium (Lu). The other rare earth metals are situated in ...
Where are alkaline earth metals found on the periodic table?
What Are the Properties of the Alkaline Earth Metals?
- Location of the Alkaline Earths on the Periodic Table. The alkaline earths are the elements located in Group IIA of the periodic table. ...
- Properties of the Alkaline Earth Metals. The alkaline earths possess many of the characteristic properties of metals. ...
- Summary of Common Alkaline Earth Properties. Typically malleable and ductile. ...
- Fun Fact. ...
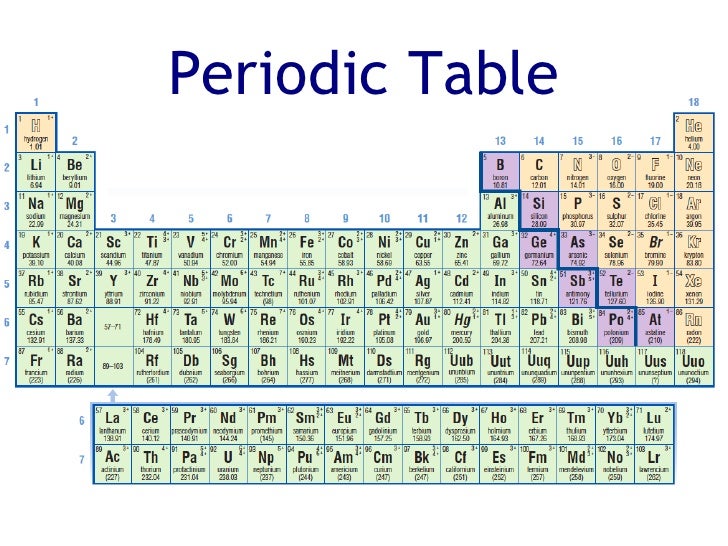
Which group of periodic table is alkali metals?
Alkali metals are present in the first group of Periodic table. First group indicates that they have only one electron in their outermost orbit. As only one electron is present in the outermost orbit, it is very easy to lose or donate this electron during a chemical reaction.
Where are the symlinks on the periodic table?
They are on the left side of the Periodic table (in group 1).
Why are alkali metals so reactive?
There are many reasons behind the reactivity of alkali metals. Let us discuss them one by one.
What metals react with water?
When these metals (Li, Na, K, Rb, Cs, Fr) react with water, they form alkalis (i.e strong base).
How many electrons are in an alkali metal?
Look, as we move down the group from top to bottom, the atomic size increases. Also alkali metals have only one electron in their outermost orbit.
Which element is the most reactive?
And hence the element with bigger atomic size (i.e Francium) is most reactive alkali metal. ( Note: Francium is a laboratory made element. It is available in a very less quantity. And hence for any practical purposes, cesium is considered as the most reactive metal in Periodic table.
Where are alkali metals located?
Alkali metals are located in group 1 on the left side of the Periodic table.
Where are transition metals found?
Transition metals are found in the middle part of the Periodic table (from Group 3 to group 11).
Where are alkaline earth metals located?
Alkaline earth metals are located on the left side of the Periodic table in group 2. Alkaline earth metals are also the reactive type of metals but they are less reactive as compared to alkali metals. All the alkaline earth metals have 2 electrons in their outermost orbit.
How many electrons are in an alkaline metal?
All the alkaline earth metals have 2 electrons in their outermost orbit.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
What is the inner transition metal?
The answer is: These elements have somewhat similar properties like that of transition metals, plus they are the elements of group 3 only, but they are placed at the bottom of the Periodic table as the inner section of group 3. Hence they are known as inner transition metals.
Which metal is the most reactive?
Alkali metals are the most reactive type of metals from the entire Periodic table of elements. As we move down the group from top to bottom in the group 1, the reactivity of alkali metals increases. List of alkali metals with atomic number, symbol and name. Atomic number. Symbol.
What color is heavy metal?
These heavy metals are displayed on the Periodic table with red color (see above image)
Where is magnesium found?
Magnesium is found in its mineral magnesite.
What happens to the metallic character of alkaline earth metals as the atomic size increases?
Down the group, the atomic size increases. And as the atomic size increases, the electron will be lost easily. So the metallic character of alkaline earth metals increases from top to bottom in a group. Read more about: Metallic character trend in periodic table.
What metals react with water?
They also react with water and moisture of the air, if they are kept open. As alkaline earth metals are reactive, they always occur in the compound form in nature. For example; Beryllium is found in its mineral beryl, chrysoberyl and phenacite. Magnesium is found in its mineral magnesite.
What is the name of the metal that does not form an alkaline solution?
See the bold word “alkaline” and “earth” in the above 2 points, you will get the exact reason why they are called alkaline earth metals. ( Note: Beryllium (Be) is the only alkaline earth metal which does not form an alkaline solution on reacting with water.
What happens to electronegativity as we move down in a group?
As we move down in a group, Electronegativity of the alkaline earth metals decreases.
What is the color of alkaline earth metals?
All the alkaline earth metals are silvery-white, shiny and somewhat reactive.
How many valence electrons does an alkaline metal have?
Alkaline earth metals are similar in the following manner. All the alkaline earth metals have 2 valence electrons in their outermost orbit. They lose these two electrons and form cation with +2 charge. As you can see above that the alkaline earth metals have 2 electrons in the outermost shell.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).

What Are Alkali Metals on The Periodic table?
Are Alkali Metals Reactive?
Which Alkali Metal Is Most Reactive?
List of Alkali Metals
Periodic Trends of Alkali Metals
Free Gift For You: Interactive Periodic Table
Summary
- In the beginning of this article, I showed you a single image which clearly shows you where are Alkali metals located on the Periodic table. They are on the left side of the Periodic table(in group 1). Then we discussed about; 1. Meaning of alkali metals 2. Reactivity of alkali metals 3. Why are alkali metals so reactive? 4. Most reactive alkali me...
Alkali Alloys
Move Materials
Principal Class
Complex Metal Alloys
Halogens
- One band of the occasional table that does not belong in the metallic loved ones are the halogens. These extremely reactive non-metal factors can be found in group of people 17 and constitute the 17th line. They can be made up of fluorine, chlorine and bromine and iodine, and as of this creating, astatine. Halogens are really unusual in the world a...
Respectable Fumes
Causes of Metal Poisoning