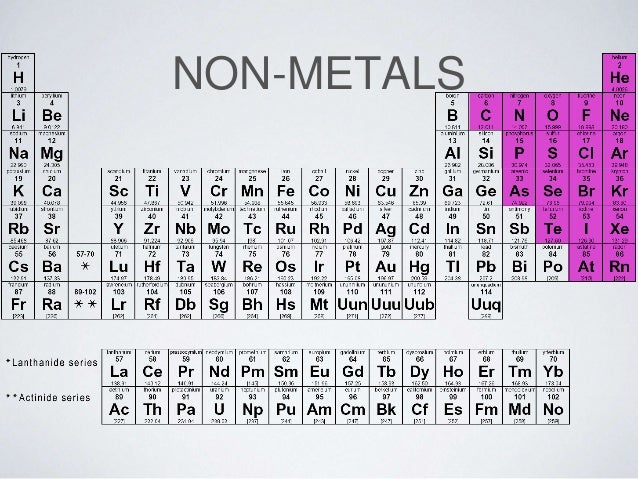
Where are non metals and metals placed on the periodic table?
The nonmetals are a group of elements located on the right side of the periodic table (except for hydrogen, which is on the top left). There are also known as non metals and non-metals.
What are the 22 non metals?
What are the twenty two non-metals? The nonmetal element group consists of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur and selenium. The elements at the top of the group are gases, but they become liquids and solids moving down the group. The halogens are fluorine, chlorine, bromine, iodine, and astatine.
Where are the most reactive metals on the periodic table?
The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. Lithium, sodium, and potassium all react with water, for example. Also asked, what are the two most reactive groups in the periodic table?
What are the 5 characteristics of nonmetals?
What are the five characteristics of nonmetals?
- for ionic/covalent bonds.
- brittle and nonmalleable.
- low melting/boiling points.
- High ionization energy and electronegativity.
- poor conductors of heat and electricity.
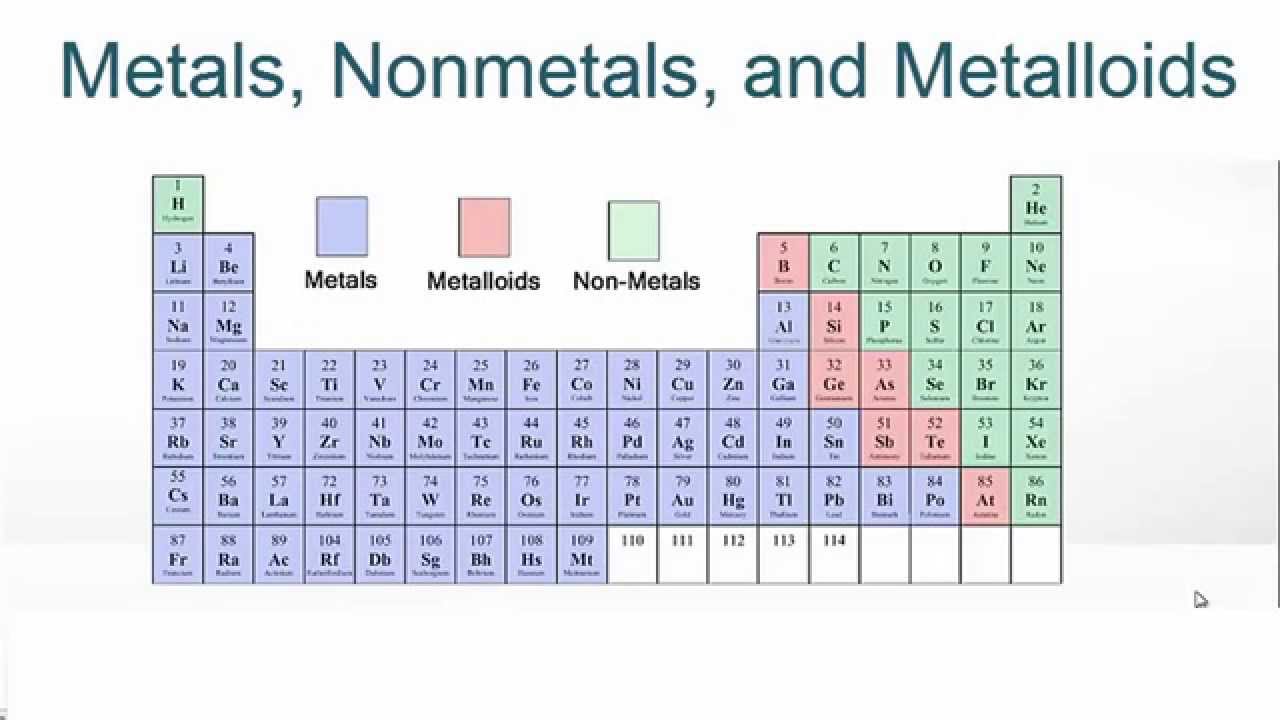
Where are nonmetals on the periodic table?
Updated February 25, 2020. The nonmetals or non-metals are a group of elements located on the right side of the periodic table ( except for hydrogen, which is on the top left). These elements are distinctive in that they typically have low melting and boiling points, don't conduct heat or electricity very well, and tend to have high ionization ...
How many elements are in the nonmetal group?
There are 7 elements that belong to the nonmetals group: Although these are the elements in the group nonmetals, there are two additional element groups that could be included, since the halogens and noble gases also are types of nonmetals.
What are the oxidation numbers of nonmetals?
Atoms of these elements have oxidation numbers of +/- 4, -3, and -2.
Is carbon a nonmetal?
Nonmetals are classified based on their properties under ordinary conditions. Metallic character isn't an all-or-nothing property. Carbon, for example, has allotropes that behave more like metals than nonmetals. Sometimes this element is considered to be a metalloid rather than a nonmetal.
What is a nonmetal?
in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. A nonmetal is simply an element that does not display the properties of a metal.
How are nonmetals separated from metals?
Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. The halogens and noble gases are nonmetals, but the nonmetal element group usually consists of the following elements: hydrogen. carbon. nitrogen. oxygen.
Where is hydrogen found in the periodic table?
The exception is hydrogen, which behaves as a nonmetal at room temperature and pressure and is found on the upper left corner of the periodic table . Under conditions of high pressure, hydrogen is predicted to behave as an alkali metal.
Is 118 a metal or a liquid?
element 118 (oganesson). This element is predicted to be a liquid but is still a nonmetal.
Is nonmetal a metal?
It doesn't look metallic, can't be made into a wire, pounded into shape or bent, doesn't conduct heat or electricity well, and doesn't have a high melting or boiling point. The nonmetals are in a minority on the periodic table, mostly located on the right-hand side of the periodic table.
What are the characteristics of nonmetals?
Nonmetals exhibit very different properties from metals. Examples of nonmetals include oxygen, chlorine, and argon. Nonmetals display some or all of the following characteristics: 1 Dull appearance 2 Usually brittle 3 Poor conductors of heat and electricity 4 Usually less dense, compared to metals 5 Usually low melting point of solids, compared with metals 6 Tend to gain electrons in chemical reactions
What are the elements in the periodic table?
Elements of the periodic table are grouped as metals, metalloids or semimetals, and nonmetals. The metalloids separate the metals and nonmetals on a periodic table. Also, many periodic tables have a stair-step line on the table identifying the element groups.
What are some examples of metalloids?
Examples of metalloids include boron, silicon, and arsenic. Metalloids have some of the properties of metals and some nonmetallic characteristics. Dull or shiny. Usually conduct heat and electricity, though not as well as metals. Often make good semiconductors.
Which element is considered a metal?
Elements to the left of the line are considered metals. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Elements to the far right of the periodic table are nonmetals. The exception is hydrogen (H), the first element on the periodic table.
What are the properties of metals?
Metals exhibit the following properties: Usually solid at room temperature (mercury is an exception) High luster (shiny) Metallic appearance. Good conductors of heat and electricity. Malleable (can be bent and pounded into thin sheets) Ductile (can be drawn into wire) Corrode or oxidize in air and seawater.
Where are metals located on the periodic table?
The metals are located on the left side of the Periodic Table.
What are metals in Periodic table?
Metals are the elements which have the tendency to donate or lose electrons to form positive ions.
How many electrons do metals lose in a chemical reaction?
The atoms or metals have generally 1, 2 or 3 electrons in the outermost orbit, and they lose these electrons during a chemical reaction.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Which group of metals is the most reactive?
They are the Alkali metals of group 1. In 1st group, as we move down from top to bottom, the reactive of metals increases. Thus the bottom most element of group 1 (i.e francium) is the most reactive metal on the Periodic table. ( Note: Francium is a laboratory made element.
Why do metals make a ringing sound?
Metals produce ringing sound when they are stuck hard. This indicates that metals are sonorous in nature.
What are the elements in group 3 to group 12?
The elements lying in group 3 to group 12 are known as Transition metals (or transition elements). Transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14. Thus these metals are known as “Transition metals”.
Where are metalloids found in the periodic table?
Also we can say that metalloids are present in the diagonal region of the p block on Periodic table.
What are elements that show some properties of metals as well as solid nonmetals called?
The elements that show some properties of metals as well as solid nonmetals are called metalloids . Metalloids look like metals, but they are not. Metalloids are brittle like solid nonmetals. Metalloids are neither conductor nor insulated. Source ( James L Marshall, Silicon, Germanium / CC BY-SA, CC BY ) The examples of metalloids are:
What are the properties of metalloids?
Let us discuss the physical properties as well as chemical properties of metalloids/semimetals.
What is the chemical reaction between halogens and metalloids?
Metalloids + Halogens = Compounds (metalloid s elements reacts with halogens and finally compounds are formed by this chemical reaction) Metalloids have different metallic allotropes as well as nonmetallic allotropes. Metalloids have the property to form glasses on oxidation and so that are used in glass manufacturing.
Why are metalloids called semiconductors?
Metalloids are called semiconductors because they are not good conductors like metals and also they are not bad conductors like nonmetals. They have the conductivity which is higher than nonmetals, but lower than metals. Hence metalloids are known as semiconductors.
How many metalloids are there?
Hence, there are total 6 known metalloids/semimetals on the Periodic table.
What is the color of the metalloids in the periodic table?
June 10, 2021 August 25, 2020 by Admin. Metalloids are located between the metals and nonmetals. The orange color on the Periodic table represents metalloids . They form a separating boundary between the metals and nonmetals. In other words, metalloids (semimetals) are located on the right side of the post transition metals and on the left side ...
