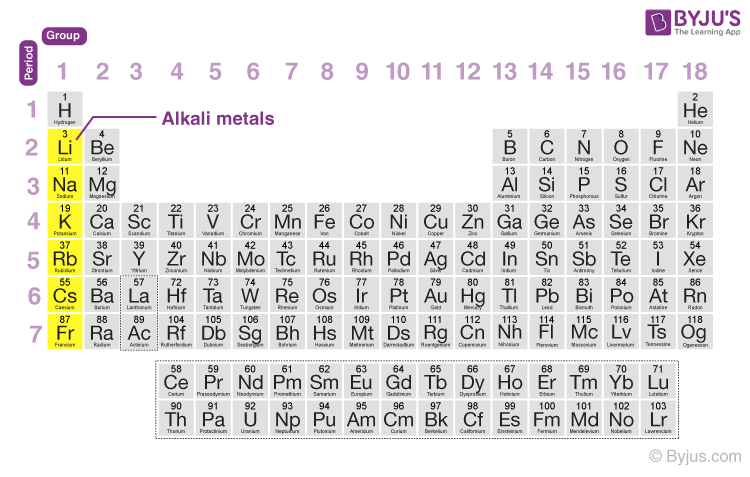
Which metal is most reactive in the periodic table?
Francium is most reactive element in periodic table. However, francium is artificial or only few quantities have produced right now, so after the francium, cesium is most reactive metal.
Where are the reactive nonmetals located?
Answer and Explanation: The most reactive nonmetals are the halogens (of group 17), which are located on the far right-hand side of the periodic table, just before the noble gases.
Where in the periodic table the most reactive and non-metals found?
The most reactive nonmetals reside in the upper right portion of the periodic table. The element fluorine is the most reactive nonmetal.
Why are the most reactive metals group 1 to the far left of the periodic table but the most reactive nonmetals Group 17 are far to the right?
Predicting the Reactivity for Nonmetals Group 17 (Halogens) are the most reactive nonmetals. This is because Halogens have 7 valence electrons in the outer shell, thus they eagerly want to gain an electron in order to complete its octet. Group 18 (Nobel Gases) are the least reactive elements.
What are reactive nonmetals?
Some nonmetals are very reactive, whereas others are not reactive at all. It depends on the number of electrons in their outer energy level. Reactive nonmetals tend to gain electrons. This explains why they cannot conduct electricity, which is a flow of electrons.
What group is the most reactive nonmetals?
Halogens are the most reactive nonmetals on the periodic table. The halogens are so reactive due to their electronic configuration. They have 7 electrons in their outermost shell and desire to gain an extra electron to complete their shell of 8 electrons.
What is reactive non-metal?
The reactivity of non-metals decreases down the group due to increases in the number of valence shells. On moving across the period the reactivity of non-metals increases. Thus, the most reactive non-metal is Flourine . Fluorine is a univalent poisonous gas that is chemically reactive.
Which group of nonmetals is the most reactive?
the halogensThe most reactive nonmetal is fluorine. Fluorine is a halogen, which is Group 17 on the periodic table, and the halogens are the most reactive nonmetals. This is because they all have one empty space in their valence electron shells.
What does a reactivity series show?
The metal reactivity series is a list of metals arranged in the order of their decreasing activities. The metals at the top of the series (K, Na, C...
Which metal is the least reactive?
Platinum is the least reactive metal. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble met...
What is metal reactivity?
Reactivity series is a list of metals arranged in decreasing order of their reactivity. Most reactive metals are at the top while the least reactiv...
Which is the most reactive metal?
alkali metal are most reactive metals. Down the reactivity increases. Francium is most reactive element in periodic table. However, francium is art...
Are non-metals Reactive or non-reactive?
Non-metal properties have a relatively low boiling point, and other non-metals are gases. Likewise, non-metals are poor heat conductors, and solid...
What is the reactivity series of metal?
The reactivity series of metals refers to the array of metals in the descending order of their reactivities. It is also known as the activity serie...
What does the reactivity series of metals depend on?
The reactivity series of metals depends on the reactivity of metals which is dependent on the atomic radius, nuclear charge, sublevel electrons arr...
How is the reactivity series of metals originated?
The reactivity of metal originated from the reactivity of metals. In the activity series of metals more reactive metal is placed on the top of the...
What is the significance of the reactivity series of metal?
The reactivity series of metals is the array of metals in the descending order of their reactivities. It helps in predicting if a metal can displac...
Which group of metals is the most reactive?
They are the Alkali metals of group 1. In 1st group, as we move down from top to bottom, the reactive of metals increases. Thus the bottom most element of group 1 (i.e francium) is the most reactive metal on the Periodic table. ( Note: Francium is a laboratory made element.
Where are metals located on the periodic table?
The metals are located on the left side of the Periodic Table.
What are metals in Periodic table?
Metals are the elements which have the tendency to donate or lose electrons to form positive ions.
How many electrons do metals lose in a chemical reaction?
The atoms or metals have generally 1, 2 or 3 electrons in the outermost orbit, and they lose these electrons during a chemical reaction.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Why do metals make a ringing sound?
Metals produce ringing sound when they are stuck hard. This indicates that metals are sonorous in nature.
What are the elements in group 3 to group 12?
The elements lying in group 3 to group 12 are known as Transition metals (or transition elements). Transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14. Thus these metals are known as “Transition metals”.
What is the most reactive metal on the periodic table?
Updated October 08, 2019. The most reactive metal on the periodic table is francium. Francium, however, is a laboratory-produced element and only minute quantities have been made, so for all practical purposes, the most reactive metal is cesium . Cesium reacts explosively with water, though it is predicted francium would react even more vigorously.
Which group of metals is the most reactive?
The most reactive metals belong to the alkali metals element group. Reactivity increases as you move down the alkali metals group. The increase in reactivity correlates to a decrease in electronegativity (increase in electropositivity.)
What Determines Reactivity?
Reactivity is a measure of how likely a chemical species is to participate in a chemical reaction to form chemical bonds. An element that is highly electronegative, such as fluorine, has an extremely high attraction for bonding electrons.
Which element is more reactive, lithium or francium?
So, just by looking at the periodic table, you can predict lithium will be less reactive than sodium, and francium will be more reactive than cesium and all of the other elements listed above it in the element group.
What happens to the atomic radius as you move down a column?
As you move down a column or group of the periodic table, the size of the atomic radius increases. For the metals, this means the outermost electrons becomes farther away from the positively-charged nucleus. These electrons are easier to remove, so the atoms readily form chemical bonds.
What is the purpose of the metal activity series?
You can use the metal activity series to predict which metal will be the most reactive and to compare the reactivity of different metals. The activity series is a chart that lists elements according to how readily the metals displace H 2 in reactions.
What is the reactivity series of metals?
The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. The data provided by the reactivity series can be used to predict whether a metal can displace another in a single displacement reaction. It can also be used to obtain information on ...
Why are metals at the top of the reactivity series powerful reducing agents?
The metals at the top of the reactivity series are powerful reducing agents since they are easily oxidized. These metals tarnish/corrode very easily. The reducing ability of the metals grows weaker while traversing down the series. The electro positivity of the elements also reduces while moving down the reactivity series of metals.
What is the long tabular form of the Reactivity Series?
Long Tabular Form of the Reactivity Series. The reactivities of metals are tabulated below (in the descending order) along with their corresponding ions. Note that the metals in Red react with cold water, those in Orange cannot react with cold water but can react with acids, and those in Blue only react with some strong oxidizing acids.
Why is hydrogen included in the reactivity series?
Despite being a non-metal, hydrogen is often included in the reactivity series since it helps compare the reactivities of the metals. The metals placed above hydrogen in the series can displace it from acids such as HCl and H 2 SO 4 (since they are more reactive).
What metals react with water?
Reaction Between Metals and Water. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding hydroxide while liberating hydrogen gas.
What can be used to predict the reactions between metals and water?
Therefore, the reactivity series of metals can be used to predict the reactions between metals and water.
How is titanium extracted?
For example, titanium is extracted from titanium tetrachloride via a single displacement reaction with magnesium. Thus, the reactivity series of metals can also be used to predict the outcome of single displacement reactions.
Which metal is the most reactive?
Alkali metals are the most reactive type of metals from the entire Periodic table of elements. As we move down the group from top to bottom in the group 1, the reactivity of alkali metals increases. List of alkali metals with atomic number, symbol and name. Atomic number. Symbol.
Where are transition metals found?
Transition metals are found in the middle part of the Periodic table (from Group 3 to group 11).
How many electrons are in an alkaline metal?
All the alkaline earth metals have 2 electrons in their outermost orbit.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
What is the inner transition metal?
The answer is: These elements have somewhat similar properties like that of transition metals, plus they are the elements of group 3 only, but they are placed at the bottom of the Periodic table as the inner section of group 3. Hence they are known as inner transition metals.
Where are alkaline earth metals located?
Alkaline earth metals are located on the left side of the Periodic table in group 2. Alkaline earth metals are also the reactive type of metals but they are less reactive as compared to alkali metals. All the alkaline earth metals have 2 electrons in their outermost orbit.
What color is heavy metal?
These heavy metals are displayed on the Periodic table with red color (see above image)
